Case of the Month ...
Case History
The patient was a 53-year-old male presented with massive upper GI bleeding. Endoscopic examination revealed a large ulcerated lesion in the greater curvature of the stomach. Both CT scan and endoscopic ultrasound revealed a 12-cm cystic gastric mass abutting the tail of the pancreas. Transgastric EUS-FNA was performed. Subsequently the patient underwent surgical excision.
Diagnosis & Discussion
click on image for larger version
Discussion
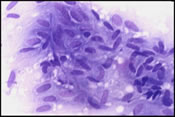
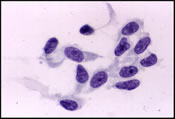
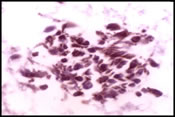
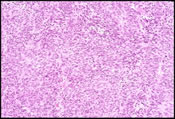
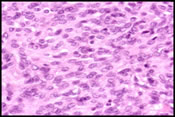
The FNA smears were moderately cellular and composed of a predominant population of spindle cells arranged in large tissue fragments, loose cohesive groups, and as isolated cells (Figure 1). The tumor cells had varying amounts of delicate and pale cytoplasm and indistinct cell membranes. The nuclei appeared elongated and cigar-shaped with blunted-end (Figure 2). There was mild to moderate nuclear atypia in terms of anisonucleosis and prominent nucleoli (Figure 3). A preliminary cytological diagnosis of spindle cell tumor was made. Immunoperoxidase stains were subsequently performed on the cellblock. The tumor cells exhibited strong c-kit (Figure 4) and vimentin immunoreactivity but negative for S-100 protein, smooth muscle actin (SMA) and cytokeratin. A final cytologic diagnosis of GIST was, therefore, made based on cytological and immunocytochemical findings.
Cytologic Diagnosis: Gastrointestinal Stromal Tumor (GIST)
Histologic Features
The patient underwent surgical excision subsequently. A 12-cm tumor originated from the gastric wall with central cystic degeneration and hemorrhage was resected. The histology sections demonstrated a proliferation of spindle cells with moderate cytological atypia and brisk mitotic activities (Figure 5 and 6). Immunoperoxidase stains were performed and the tumor cells were strongly immunoreactive for c-kit and CD34, but negative for S-100, SMA and desmin.
Discussion
GISTs are thought to arise from the interstitial cells of Cajal or multipotent cells that can differentiate into Cajal cells (1). They occur in middle-aged and elderly patients. Female is more commonly affected than male (1). About 10-30% of GISTs are malignant. Seventy percent of GISTs arise from the stomach, followed by the small bowel (20%) and esophagus and colon (1).
The differential diagnosis of GISTs includes smooth muscle neoplasm, solitary fibrous tumor, and sarcomatous carcinoma. The use of immunocytochemistry is helpful in the differential diagnosis. Table 1 summarized the immunophenotype of each lesion. About 80-100% of GISTs demonstrate strong immunoreactivity for c-kit (2).
The aspirate material of EUS-FNA is usually obtained through the wall of the GI tract. Fragments of normal gastric or bowel wall (Figure 7) can sometimes be mistaken as GIST tumor. The former tends to appear bland and lack c-kit reactivity. On the other hand, one may also confuse rare clusters of spindle cells from a GIST with the muscularis propria of the bowel.
Cytomorphology alone cannot predict the biologic behavior of GIST. Some of the poor prognostic variables include distal lesion, tumor larger than 5 cm, presence of mitotic figures, and more than 10% of tumor cells demonstrated positive Ki67 staining. Recently, the presence of c-kit mutations has been found to be associated with poor prognosis in patients with GIST (3-8) . A recent study by Li and associates demonstrated the utility of detection of c-kit mutation in the FNA specimen (9). Although the presence of c-kit mutation in GISTs was strongly predictive of malignant behavior, they found some malignant GISTs did not demonstrated c-kit mutations. However, their study is limited by amplifying only a segment of c-kit exon 11, as cases of malignant GISTs with mutations in either exon 9 or 13, but not in exon 11, have been reported (10).
Table 1 Differential Diagnosis of GIST based on Immunophenotyping.
References
1. Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol. 1999;30:1213-20
2. Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M. CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod Pathol 1998; 11:728-34
3. Sakurai S, Fukasawa T, Chong JM, Tanaka A, Fukayama M. C-kit gene abnormalities in gastrointestinal stromal tumors (tumors of interstitial cells of Cajal. Japan J Cancer Res 1999; 90:1321-8.
4. Taniguchi M, Nishida T, Hirota S, et al. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 1999; 59:4297-300.
5. Seidal T, Edvardsson H. Expression of c-kit (CD117) and Ki67 provides information about the possible cell of origin and clinical course of gastrointestinal stromal tumours. Histopathology 1999; 34:416-24.
6. Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol 1999; 154:53-60.
7. Nakahara M, Isozaki K, Hirota S, et al. A novel gain-of-function mutation of c-kit gene in gastrointestinal stromal tumors. Gastroenterol 1998; 115:1090-5.
8. Ernst SI, Hubbs AE, Przygodzki RM, Emory TS, Sobin LH, O'Leary TJ. KIT mutation portends poor prognosis in gastrointestinal stromal/smooth muscle tumors. Lab Invest0 1998; 78:1633-6.
9. Li SQ, O'Leary TJ, Sobin LH, Erozan YS, Rosenthal DL, Przygodzki RM. Analysis of KIT mutation and protein expression in fine needle aspirates of gastrointestinal stromal/smooth muscle tumors. Acta Cytol 2000; 44:981-6.
10. Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000; 156:791-5.