Case of the Month ...
Case History
A 48 year-old woman presented with epigastric fullness, bloating, and right upper back pain with associated pruritis, light-colored stools, and dark-colored urine. On physical examination, she was jaundiced with scleral icterus and, on palpation, had mild fullness in the right upper quadrant. Computed tomography (CT) with contrast of the abdomen revealed a 2.5 cm mass in the head of the pancreas, suggestive of adenocarcinoma. Also noted on CT was a 4.3 cm avidly enhancing mass in the right adrenal gland, and chemical evaluation was suggested to rule out a functional adrenal mass. Plasma renin, aldosterone, normetanephrine, and metanephrine were within normal limits, and a CT guided Fine needle aspiration of the right adrenal mass was performed. Subsequently, the patient underwent a Whipple resection and right adenalectomy. Pathology revealed at moderately differentiated pancreatic adenocarcinoma of the head of the pancreas and a 4.5 cm, 32.9 gram, encapsulated, variegated green-brown tumor of the right adrenal gland.
Diagnosis & Discussion
click on image for larger version
Figure 1
Image Figures:
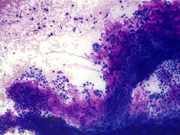
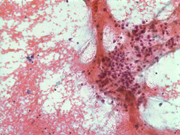
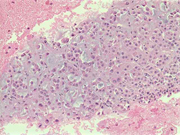
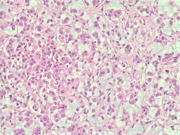
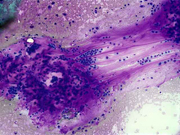
Figure 1: FNA cytology smear of adrenal gland, Diff-Quik stain, X 100
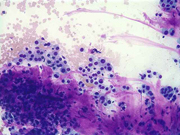
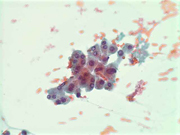
Figure 2: FNA cytology smear of adrenal gland, Diff-Quik stain, X 100
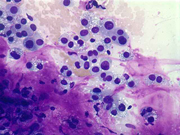
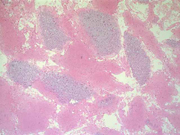
Figure 3: FNA cytology smear of adrenal gland, Diff-Quik stain, X 200
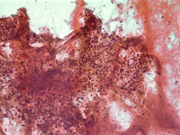
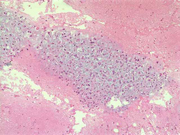
Figure 4: FNA cytology smear of adrenal gland, Diff-Quik stain, X 400
Figure 5: FNA cytology smear of adrenal gland, Papanicolaou stain, X 100
Figure 6: FNA cytology smear of adrenal gland, Papanicolaou stain, X 200
Figure 7: FNA cytology smear of adrenal gland, Papanicolaou stain, X 400
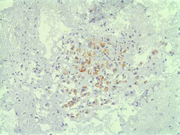
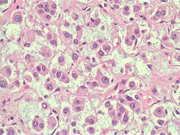
Figure 8: FNA cell block, H & E, X 40
Figure 9: FNA cell block, H & E, X 100
Figure 10: FNA cell block, H & E, X 200
Figure 11: FNA cell block, Melan A immunoperoxidase stain, X 200
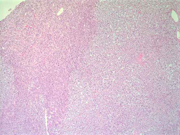
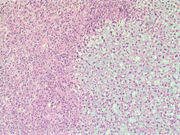
Figure 12: Surgical resection of adrenal gland, H & E, X 40
Figure 13: Surgical resection of adrenal gland, H & E, X 100
Figure 14: Surgical resection of adrenal gland, H & E, X 200
Figure 15: Surgical resection of adrenal gland, H & E, X 400Diagnosis: Myxoid adrenal cortical adenoma
Cytologic Findings:
- Loose aggregates and individual cells
- Round to polygonal cells with indistinct cell borders
- Uniform, round to oval nuclei with small nucleoli
- Abundant vacuolated cytoplasm
- Myxoid background
- Absence of mitotic figures and necrosis
Discussion: Adrenal cortical adenomas and carcinomas are rare tumors but with the use of high resolution imaging, such as CT and MRI, incidentally discovered adrenal masses have become a common finding. The diagnostic evaluation of incidental adrenal lesions includes imaging modalities such as CT with and without contrast, MRI and PET, hormonal evaluation, and FNA in certain clinical settings, particularly to exclude metastasis. In up to 20% of cases, these incidental lesions may cause abnormal hormone secretion without obvious clinical manifestations, while nonfunctioning incidental adrenal lesions less than 5 cm are likely to be benign.
Distinguishing an adrenal cortical adenoma from carcinoma is a well-known diagnostic challenge in surgical pathology, and thus, in fine needle aspiration cytology. Proposed criteria correlating with subsequent malignant behavior include the combination of three or more of the following: high nuclear grade, greater than 5 mitoses per 50 high-power fields, atypical mitotic figures, less than 25% of tumor cells are clear cells, diffuse architecture, necrosis, venous invasion, sinusoidal invasion, and capsular invasion. Furthermore, size (less than 6 cm), weight (less than 100 gram), and a MIB-1/Ki-67 labeling index of 5-20% have been proposed to favor the diagnosis of adenoma.
Myxoid change in adrenal cortical adenomas and carcinomas is an extremely rare but reported finding. The tumors display a range of architectural patterns with varied myxoid composition, ranging from 10% to 95%. The myxoid areas stain positively with Alcian blue, and focal positivity with periodic acid-Schiff and mucicarmine has been noted in few cases. Positive immunohistochemical staining for alpha-inhibin, synaptophysin, and vimentin is present in these tumors, and focal cytokeratin staining has been noted. The differential diagnosis of these myxoid adrenal lesions includes metastatic adenocarcinoma, retroperitoneal soft tissue neoplasms with myxoid change, and chordoma.
In the above case, the surgical resection specimen revealed an encapsulated myxoid adrenal neoplasm with no features of malignancy such as high nuclear grade, mitoses, atypical mitoses, necrosis, or capsular and vascular invasion. Immunohistochemistry was positive for alpha-inhibin and melan-A, and MIB-1 staining was negative. Taken together, these features are consistent with the diagnosis of a myxoid adrenal cortical adenoma.
References:
1. Geisinger KR, Stanley MW, Raab SS, Silverman JF, Abati A. Modern Cytopathology. Churchill Livingstone, Philadelphia, 2004, 621-9.
2. DeLellis FA, Lloyd RV, Heitz PU, Eng C. WHO Classification Tumours of Endocrine Organs. IARC Press, Lyon, 2004, 135-46.
3. Brown FM, Gaffey TA, Wold LE, Lloyd RV. Myxoid neoplasms of the adrenal cortex: A rare histologic variant. AJSP, 2000, 24(3): 396-401.
4. Bollito ER, Papotti M, Porpiglia F, Terzolo M, Cracco CM, Cappia S, Gubetta L, Mikuz G. Myxoid adrenocortical adenoma with a pseudoglandular pattern. Virchows Arch, 2004, 445: 414-418.
5. Fine SW, Pindzola JA, Uhlman EJ, Epstein JI. Pathologic quiz case: a 64-year-old man with an adrenal mass. Myxoid adrenal cortical neoplasm. Arch Pathol Lab Med, 2005, 129(4): 541-2.
6. Karim RZ, Wills EJ, McCarthy SW, Scolyer RA. Myxoid variant of adrenocortical carcinoma: report of a unique case. Pathol Int, 2006, 56(2): 89-94.
7. Suresh B, Kishore TA, Albert AS, Joy A. Myxoid adrenal cortical carcinoma: a rare variant of adrenocrotical carcinoma. Indian J Med Sci, 2005, 59(11): 505-7.Acknowledgment: Submitted by Kelly M. Hornaman, M.D. and Momin T. Siddiqui, M.D., FIAC. Emory University School of Medicine, Atlanta, GA.