Case of the Month ...
Case History
15-year-old female presents with a 9 cm abdominal mass that appears to arise from the pancreas. CT-guided aspiration of mass was performed.
Diagnosis & Discussion
click on image for larger versionImage Figs:
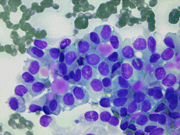
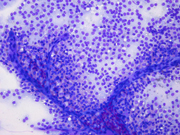
- Figure 1: FNA direct smear of mass, Diff-Quik stain, x100
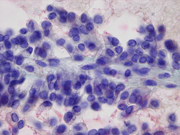
- Figure 2: FNA direct smear of mass, Diff-Quik stain, x400
- Figure 3: FNA direct smear of mass, Papanicolaou stain, x400
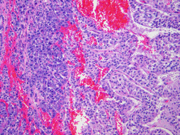
- Figure 4: Surgical resection of mass, H&E stain, x100
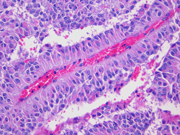
- Figure 5: Surgical resection of mass, H&E stain, x200
Questions:
- What is the diagnosis?
- Intraductal papillary mucinous neoplasm
- Acinar cell carcinoma
- Solid pseudopapillary tumor
- Pancreatic endocrine neoplasm
- Which of the following is not a characteristic cytologic feature of this tumor?
- Intranuclear inclusions
- Polarized nuclei with nuclei positioned away from the papillary or pseudopapillary core
- Inconspicuous nucleoli
- Hyaline globules
- Which of the following is false about this tumor?
- Typically large, friable, and hemorrhagic
- Poor prognosis
- Radiologically and grossly well-demarcated
- Usually presents in young women
- Which of the following immunostains is expected to always be negative in this tumor?
- Chromogranin
- Synaptophysin
- Beta-catenin
- CD10
Discussion:Solid pseudopapillary tumor of pancreas (SPT) is a rare low-grade malignant epithelial neoplasm accounting for 1%–2% of all pancreatic neoplasms. SPT occurs predominantly in young women and is often asymptomatic. It may arise anywhere within the pancreas. Presenting symptoms may include abdominal pain, palpable mass, dyspepsia, bloating, or obstructive jaundice. Most patients have long-term survival with surgical resection of the primary tumor and metastases. However, SPT with apparent malignant transformation and a highly aggressive course have been described.Radiologically, SPT is usually a well-circumscribed multilocular cystic mass with solid portions partially replacing the pancreas, findings that are suggestive but not specific for this tumor. [9] Irregular calcifications may be seen. On CT scan and ultrasound, SPT shows solid areas as well as areas of cystic degeneration, hemorrhage, or necrosis.
Grossly, SPTs are well-demarcated, sometimes partially encapsulated tumors that are red-tan, soft, friable, and hemorrhagic, often with cystic changes. Most cases are large at presentation with a mean diameter of 9 to 10 cm, although the size may range from 1.5 cm to 30 cm.
Cytomorphologically, smears from fine-needle aspirations (FNA) are cellular and show single cells, clusters, and branching, papillary (pseudopapillae) fragments. The papillary structures typically demonstrate a central fibrovascular core and myxoid fibrillary stroma. The fibrovascular cores are covered by one or more layers of cells, which may be polarized with the nuclei positioned away from the core. The neoplastic cells are monomorphic and round to oval with fine, evenly distributed chromatin and inconspicuous nucleoli. Nuclear grooves may also be present. Hyaline globules are another characteristic feature of SPT and may be either extracellular or within gland-like/acinar structures.
Histologically, SPT shows a solid growth pattern of uniform cells with abundant small vessels. Degenerative changes occur in areas away from vessels resulting in the formation of pseudopapillae, cuffs of neoplastic cells clinging to the blood vessels. Other degenerative changes include macrophages, foreign-body giant cells, hemorrhage, cholesterol clefts, fibrosis, and calcifications. Similar to aspiration specimens, nuclei are sometimes oriented away from the vessels. Eosinophilic diastase resistant PAS-positive hyaline globules are a characteristic features. Mitotic figures are rare. A diffuse growth pattern with extensive tumor necrosis, high mitotic rate, and the presence of an undifferentiated component, such as sarcomatoid carcinoma, are associated with an aggressive clinical behavior.
Immunohistochemically, SPTs are typically positive for vimentin, CD10, neuron-specific enolase, CD56, PR, alpha-1-antitrypsin, and alpha-1-antichymotrypsin. Beta-catenin is also commonly positive. SPTs are negative for chromogranin, trypsin, and chymotrypsin and variably express cytokeratins and synaptophysin.
The differential diagnosis includes pancreatic endocrine neoplasm (PEN), acinar cell carcinoma, intraductal papillary mucinous neoplasm (IPMN), and pseudocyst. PEN aspiration biopsies are cellular and often contain loosely cohesive cell groups and rosette-like structures, but lack papillary structures. The tumor cells are uniform, often plasmacytoid, and contain stippled, salt-and-pepper chromatin with inconspicuous nucleoli. Immunohistochemically, most PENs are positive for chromogranin, synaptophysin, and NSE. Acinar cell carcinoma has a predilection for older males, displays an ill-defined gross appearance, lacks pseudopapillary structures, and is composed of round to oval cells with distinct nucleoli. Acinar cell carcinomas are positive for trypsin, chymotrypsin, and cytokeratins and negative for neuroendocrine markers, though focal immunoreactivity with neuroendocrine markers can be observed. IPMNs are tumors involving the main pancreatic duct and/or branch ducts. Aspirations often contain viscous mucin and may contain cell clusters arranged in true papillae and cells with intracellular mucin. Cytologic atypia may range from mild to severe (in situ carcinoma). IPMNs are positive for keratins, including AE1/AE3, CAM5.2, CK7, and variably for CK20. Pseudocysts are distinguished by different clinical and microscopic findings. Pseudocysts usually occur in patients with a history of pancreatitis, i.e., middle-aged or older men. Microscopically, they are a collection of necrotic and hemorrhagic material surrounded by a fibrous capsule that lacks an epithelial lining.
Answers to the questions:
- C
- A
- B
- A
REFERENCES
1. Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20 Suppl 1:S94-112.
2. Imaoka H, Yamao K, Bhatia V, Shimizu Y, Yatabe Y, Koshikawa T, Kinoshita Y. Rare pancreatic neoplasms: the utility of endoscopic ultrasound-guided fine-needle aspiration-a large single center study. J Gastroenterol 2009;44(2):146-53.
3. Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy 2008;40(3):200-3.
4. Adamthwaite JA, Verbeke CS, Stringer MD, Guillou PJ, Menon KV. Solid pseudopapillary tumour of the pancreas: diverse presentation, outcome and histology. Jop 2006;7(6):635-42.
5. Sperti C, Berselli M, Pasquali C, Pastorelli D, Pedrazzoli S. Aggressive behaviour of solid-pseudopapillary tumor of the pancreas in adults: a case report and review of the literature. World J Gastroenterol 2008;14(6):960-5.
6. Yang F, Jin C, Long J, Yu XJ, Xu J, Di Y, Li J, Fu de L, Ni QX. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg 2009;198(2):210-5.
7. Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, Guiter G, Stanley MW. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol 2004;121(5):654-62.
8. Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29(4):512-9.
9. Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma P, Insabato L. Solid-pseudopapillary tumor of the pancreas: a neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol 2002;27(6):325-34.
10. Matos JM, Grutzmann R, Agaram NP, Saeger HD, Kumar HR, Lillemoe KD, Schmidt CM. Solid pseudopapillary neoplasms of the pancreas: a multi-institutional study of 21 patients. J Surg Res 2009;157(1):e137-42.
11. Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. Jop 2006;7(1):131-6.
12. Bellizzi AM, Stelow EB. Pancreatic cytopathology: a practical approach and review. Arch Pathol Lab Med 2009;133(3):388-404.
13. Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol 2000;17(1):66-80.
14. Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Pantazopoulou A, Dappola V. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas: a case report and literature review. World J Gastroenterol 2007;13(38):5158-63.
15. Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y and others. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res 2001;61(23):8401-4.
16. Chatzipantelis P, Salla C, Konstantinou P, Karoumpalis I, Sakellariou S, Doumani I. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: a study of 48 cases. Cancer 2008;114(4):255-62.
17. Labate AM, Klimstra DL, Zakowski MF. Comparative cytologic features of pancreatic acinar cell carcinoma and islet cell tumor. Diagn Cytopathol 1997;16(2):112-6.
18. Layfield LJ, Cramer H. Fine-needle aspiration cytology of intraductal papillary-mucinous tumors: a retrospective analysis. Diagn Cytopathol 2005;32(1):16-20.
19. Hruban RH, Pitman MB, Klimstra DS. Tumors of the Pancreas. Silverberg SG, editor. Washington, DC: American Registry of Pathology; 2007. 422 p.
Case contributed by
John Crapanzano, MD
Associate Professor
Columbia Presbyterian Hospital
Department of Pathology