Case of the Month ...
Case History
CT-guided fine needle aspiration of a pancreatic mass in a 56-year-old man.
Diagnosis & Discussion
click on image for larger version
Image Figs:
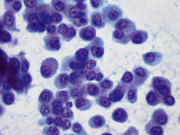
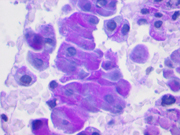
- Figure 1: FNA direct smear of mass, Papanicolaou stain, x200
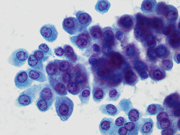
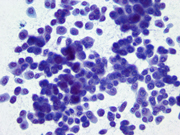
- Figure 2: FNA direct smear of mass, Papanicolaou stain, x400
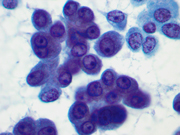
- Figure 3: FNA direct smear of mass, Papanicolaou stain, x400
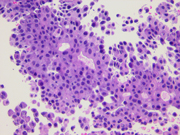
- Figure 4: FNA direct smear of mass, Papanicolaou stain, x600
- Figure 5: Core biopsy histology of mass, H&E stain, x200
- Figure 6: Core biopsy histology of mass, dPAS stain, x600
Questions:
- What is the diagnosis?
- Ductal adenocarcinoma
- Acinar cell carcinoma
- Solid pseudopapillary tumor
- Pancreatic neuroendocrine tumor
- Which of the following is a characteristic cytologic feature of this tumor?
- Clumped chromatin
- Polarized nuclei with nuclei positioned away from the papillary or pseudopapillary core
- Inconspicuous nucleoli
- Hyaline globules
- Which of the following is true about this tumor?
- Typically large, friable, and hemorrhagic
- Poor prognosis
- Usually radiologically and grossly ill-demarcated
- Typically presents in young women
- Which of the following immunostains is most likely to be negative in this tumor?
- Chromogranin
- Chymotrypsin
- Trypsin
- Beta catenin
Discussion:
Acinar cell carcinoma (ACC) of the pancreas is an uncommon tumor, representing 1-2% of pancreatic neoplasms. [1,2] Typically, it occurs in older men with the average age being 58 years. [1,2] Presenting symptoms include abdominal pain, nausea, vomiting, weight loss, and rarely jaundice. [1,3] Approximately 10-15% of patients develop lipase hypersecretion syndrome, which consists of serum lipase elevation accompanied by subcutaneous fat necrosis and polyarthralgia. [1,2] The 5-year survival rate is 5-10%. [2] ACCs are aggressive tumors with approximately 50% of patients having metastatic disease at presentation. [1] Five-year survival is 6%, and median survival is 18-19 months. [1]
Radiologically, ACC shows distinctive CT and MRI findings. [3] Their appearance is usually well-defined, oval, round, or lobulated. They may be either completely solid or show a mix of solid and cystic areas. Solid tumor enhances less than surrounding normal pancreas, and cystic masses show homogeneous enhancement of the peripheral solid components.
ACC may arise anywhere in the pancreas, [1] but most involve the pancreatic head. [2] Grossly, ACCs are usually large, averaging 8-10 cm, and circumscribed. [1,2] They are soft, tan to red, fleshy tumors. [1] Necrosis and hemorrhage may be present. [3]
Cytomorphologically, smears from fine-needle aspirations (FNAs) of ACC are cellular and comprised of somewhat polygonal, monomorphic tumor cells arranged predominantly in loose clusters that may exhibit either vague or prominent acinar formations or as single cells. [2,4,5] Nuclei may be central or eccentric, which imparts a plasmacytoid appearance. Nuclei are round to oval with clumped chromatin and prominent nucleoli. A salt-and-pepper chromatin pattern may also be observed. [5] Numerous stripped neoplastic nuclei have also been described. [4] Cytoplasm is scant to abundant and typically granular.
Histologically, ACCs show several different architectural patterns with the most common being the solid and acinar patterns. [1,3,6] The acinar pattern demonstrates minute lumina, whereas the solid pattern shows solid sheets and nests of cells without evident lumen formation. Other architectural patterns include the glandular pattern, which is comprised of dilated acinar structures, and the trabecular pattern. Cytoplasmic zymogen granules are positive for periodic acid Schiff (PAS) and are resistant to diastase digestion. [1,3,5,6] Mitoses are usually easily detectable. [1]
Immunohistochemically, ACCs are typically positive for cytokeratin, including pancytokeratin [4] and CAM5.2 [3] , trypsin, and chymotrypsin. [1,3,5] Commonly, these tumors demonstrate focal neuroendocrine differentiation with scattered cells that stain for chromogranin or synaptophysin. [1,3]
The primary differential diagnosis is pancreatic neuroendocrine tumor (NET). Cytomorphologically, FNAs of pancreatic NETs are cellular and usually comprised of uniform, round, polygonal, or plasmacytoid cells with scant to abundant cytoplasm that are arranged as single cells and loose clusters. [2,4,5,7] Rosette or acinar-type formations are often present [2,4,7] and may be difficult or impossible to distinguish from the acinar architecture seen in ACC. Generally, the nuclei of pancreatic NETs exhibit a salt-and-pepper chromatin pattern and inconspicuous nucleoli, [2,4] although the chromatin may be finely granular or clumped, and nucleoli may be prominent. [2,4] The immunohistochemical profile of pancreatic NET typically shows positive staining with pancytokeratin, [2,4] chromogranin and/or synaptophysin, [1,2,4,7] CD56, and NSE. [1,7] Scattered cells may be positive for trypsin or chymotrypsin, but if more than 25% of the neoplastic cells in a predominantly neuroendocrine tumor express markers of acinar differentiation, the neoplasm should be classified as a mixed acinar-endocrine carcinoma. [1] Solid pseudopapilllary tumor (SPT) may be another diagnostic consideration. FNAs from SPT are cellular and show single cells, clusters, and branching, papillary (pseudopapillae) fragments that typically demonstrate a central fibrovascular core and myxoid, fibrillary stroma. [2,8-10] The fibrovascular cores are covered by one or more layers of cells, which may be polarized with the nuclei positioned away from the core. [10] The neoplastic cells are monomorphic and round to oval with fine, evenly distributed chromatin and inconspicuous nucleoli. [8-11] Nuclear grooves may also be present. [2,10] Hyaline globules are another characteristic feature of SPT and may be either extracellular or within gland-like/acinar structures. [2,10] Immunohistochemically, SPTs are typically positive for vimentin, CD10, neuron-specific enolase, CD56, PR, alpha-1-antitrypsin, alpha-1-antichymotrypsin, and beta-catenin. [1,9,10,12-17] and negative for chromogranin, [1,16,18] trypsin, and chymotrypsin. [1,16] They variably express cytokeratins and synaptophysin. [1,13,16] Lastly, pancreatic ductal adenocarcinoma may be in the differential diagnosis, but these tumors generally show features typical of adenocarcinoma, including frankly malignant cells with coarse chromatin, prominent nucleoli, and vacuolated cytoplasm. Cell arrangements include 3-D clusters, loosely cohesive clusters, and single cells.
Answers to the questions:
- B
- A
- B
- D
1. Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20 Suppl 1:S94-112.
2. Bellizzi AM, Stelow EB. Pancreatic cytopathology: a practical approach and review. Arch Pathol Lab Med 2009;133(3):388-404.
3. Tatli S, Mortele KJ, Levy AD, Glickman JN, Ros PR, Banks PA, Silverman SG. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol 2005;184(2):511-9.
4. Stelow EB, Bardales RH, Shami VM, Woon C, Presley A, Mallery S, Lai R, Stanley MW. Cytology of pancreatic acinar cell carcinoma. Diagn Cytopathol 2006;34(5):367-72.
5. Labate AM, Klimstra DL, Zakowski MF. Comparative cytologic features of pancreatic acinar cell carcinoma and islet cell tumor. Diagn Cytopathol 1997;16(2):112-6.
6. Samuel LH, Frierson HF, Jr. Fine needle aspiration cytology of acinar cell carcinoma of the pancreas: a report of two cases. Acta Cytol 1996;40(3):585-91.
7. Chang F, Chandra A, Culora G, Mahadeva U, Meenan J, Herbert A. Cytologic diagnosis of pancreatic endocrine tumors by endoscopic ultrasound-guided fine-needle aspiration: a review. Diagn Cytopathol 2006;34(9):649-58.
8. Jani N, Dewitt J, Eloubeidi M, Varadarajulu S, Appalaneni V, Hoffman B, Brugge W, Lee K, Khalid A, McGrath K. Endoscopic ultrasound-guided fine-needle aspiration for diagnosis of solid pseudopapillary tumors of the pancreas: a multicenter experience. Endoscopy 2008;40(3):200-3.
9. Bardales RH, Centeno B, Mallery JS, Lai R, Pochapin M, Guiter G, Stanley MW. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid-pseudopapillary tumor of the pancreas: a rare neoplasm of elusive origin but characteristic cytomorphologic features. Am J Clin Pathol 2004;121(5):654-62.
10. Pettinato G, Di Vizio D, Manivel JC, Pambuccian SE, Somma P, Insabato L. Solid-pseudopapillary tumor of the pancreas: a neoplasm with distinct and highly characteristic cytological features. Diagn Cytopathol 2002;27(6):325-34.
11. Imaoka H, Yamao K, Bhatia V, Shimizu Y, Yatabe Y, Koshikawa T, Kinoshita Y. Rare pancreatic neoplasms: the utility of endoscopic ultrasound-guided fine-needle aspiration-a large single center study. J Gastroenterol 2009;44(2):146-53.
12. Adamthwaite JA, Verbeke CS, Stringer MD, Guillou PJ, Menon KV. Solid pseudopapillary tumour of the pancreas: diverse presentation, outcome and histology. Jop 2006;7(6):635-42.
13. Santini D, Poli F, Lega S. Solid-papillary tumors of the pancreas: histopathology. Jop 2006;7(1):131-6.
14. Klimstra DS, Wenig BM, Heffess CS. Solid-pseudopapillary tumor of the pancreas: a typically cystic carcinoma of low malignant potential. Semin Diagn Pathol 2000;17(1):66-80.
15. Salla C, Chatzipantelis P, Konstantinou P, Karoumpalis I, Pantazopoulou A, Dappola V. Endoscopic ultrasound-guided fine-needle aspiration cytology diagnosis of solid pseudopapillary tumor of the pancreas: a case report and literature review. World J Gastroenterol 2007;13(38):5158-63.
16. Tang LH, Aydin H, Brennan MF, Klimstra DS. Clinically aggressive solid pseudopapillary tumors of the pancreas: a report of two cases with components of undifferentiated carcinoma and a comparative clinicopathologic analysis of 34 conventional cases. Am J Surg Pathol 2005;29(4):512-9.
17. Tanaka Y, Kato K, Notohara K, Hojo H, Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y and others. Frequent beta-catenin mutation and cytoplasmic/nuclear accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer Res 2001;61(23):8401-4.
18. Yang F, Jin C, Long J, Yu XJ, Xu J, Di Y, Li J, Fu de L, Ni QX. Solid pseudopapillary tumor of the pancreas: a case series of 26 consecutive patients. Am J Surg 2009;198(2):210-5.
Contributed by:
John Crapanzano, MD
Columbia Presbyterain Medical Center, New York, NY