Case of the Month ...
Clinical History:
The patient was a 44 year-old man who presented with memory impairment and mood disturbances. He was found to have elevated serum calcium (11.4 mg/dL) accompanied by elevated parathyroid hormone (342 pg/mL). His past medical history was significant for pheochromocytoma and he underwent adrenalectomy for the same 19 years ago. On imaging, in addition to parathyroid enlargement (1.1cm), the patient also had multiple thyroid nodules, largest measuring 2.7 cm in the right lobe and 1.6 cm in the left lobe. In addition there were multiple enlarged right cervical lymph nodes ranging from 0.8 to 2.8 cm. FNAs of the thyroid nodules and cervical lymph node were performed. Total thyroidectomy and bilateral parathyroidectomy were done after the FNA diagnosis and the patient remains well 2 months post-surgery. The histopathological examination confirmed the cytological diagnosis and also extensive lymph node involvement by metastatic disease. The AJCC stage was pT2N1b amounting to at least Stage IVA disease. The results of genetic testing are discussed at the end of discussion.
Diagnosis & Discussion
click on image for larger version
Image Figs:
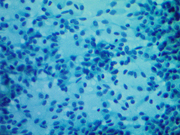
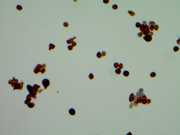
Fig 1: FNA cytology, Right thyroid nodule, Diff Quik , 400X,
Fig 2: FNA cytology, Right thyroid nodule, Pap, 400X
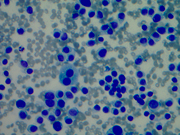
Fig 3: FNA cytology, Left thyroid nodule, Diff Quik ,400X
Fig 4: FNA cytology, Left thyroid nodule, Pap, 200X
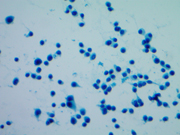
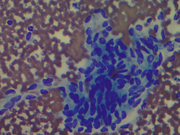
Fig5: FNA cytology, Right thyroid nodule, Calcitonin immunoperoxidase stain, 400X
Fig6: FNA cytology, Left thyroid nodule, Calcitonin immunoperoxidase stain, 400X
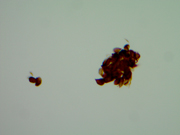
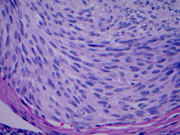
Fig 7: Right thyroid nodule, 400X, H&E
Fig 8: Left thyroid nodule, 400X, H&E
Questions:
- What are the diagnoses on the right and left side FNA?
- Medullary thyroid carcinoma
- Thyroid lymphoma
- Hurthle cell carcinoma
- Papillary thyroid carcinoma
- Which syndrome is associated with this presentation?
- MEN1
- MEN2A
- FAP
- Von Recklinghausen syndrome
- This syndrome is associated with which gene mutation?
- BRAF
- APC
- RET
- C-MYC
- Which immunohistochemical stain is used to confirm this diagnosis?
- Thyroglobulin
- Calcitonin
- CK7
- TTF-1
- What is the pre-neoplastic lesion associated with this tumor?
- Hashimoto's thyroiditis
- Papillary microcarcinoma
- C-cell hyperplasia
- Grave's disease
Discussion:
Medullary thyroid carcinomas (MTC) are distinct group of thyroid carcinomas that arise from the parafollicular C-cells. C-cells are the neuroendocrine cell rests in the thyroid gland which are of neural crest origin. MTCs comprise 5-10% of thyroid malignancies. They are sporadic in 75% cases and when familial are associated with Multiple Endocrine Neoplasia syndromes (MEN2A and MEN2B) and Familial Medullary Carcinoma (FMTC) syndrome. Familial tumors present at a younger age and are more frequently multicentric or bilateral. The familial MTCs arise more commonly on a background of C-cell hyperplasia, which can serve as an important diagnostic clue. The familial MTCs are associated with a germline mutation in the RET proto-oncogene and are often associated with other endocrine tumors depending on the site to the mutation in the RET gene.
The common histological types of MTC are plasmacytoid and spindle cells type. The tumor cells show a sheet-like, nested, trabecular or insular pattern with frequent presence of amyloid in the stroma. The cells show typical neuroendocrine features with a hyperchromatic nucleus and a powdery salt and pepper chromatin. The tumors measuring less than 1 cm are termed as medullary microcarcinomas. On cytology smears, the classical findings are a cellular smear showing loosely cohesive cells of either plasmacytoid, polygonal or spindle cell morphology with a round, eccentric or spindle shaped nucleus with salt-pepper chromatin and azurophilic cytoplasmic granules. Amyloid may be present in variable amounts. Intra-nuclear or intracytoplasmic mucin vacuoles can be seen in a fraction of cases but are not of much diagnostic importance. The most specific immunohistochemical stain for the diagnosis of medullary thyroid carcinoma is calcitonin. About 80-90% of these show diffuse positive cytoplasmic staining. Some cases may show only focal positivity. If material is insufficient for immunohistochemistry the diagnosis and be aided by serum calcitonin levels. The tumors are also positive for other neuroendocrine markers like Chromogranin A and synaptophysin. Carcinoembryonic antigen (CEA), thyroid transcription factor-1 (TTF-1) and cytokeratin 7 (CK7) are other useful markers. The tumors are typically negative for thyroglobulin. There can be a wide variation in the cytomorphology of these tumors including the follicular variant, papillary variant, oncocytic variant, small cell variant, giant cell variant, clear cell variant, melanotic variant, squamous variant, encapsulated variant, and paraganglioma like variants, causing difficulties in cytodiagnosis of these tumors. Therefore it is essential to confirm the diagnosis by immunohistochemistry when in doubt.
he unusual feature in cytology observed in this case was the presence of different cytomorphology of the right and left nodules. This is an interesting cytologic feature that has not been reported before. The right thyroid nodules showed a pure spindle cell morphology with blunt ended spindle shaped nuclei having salt and pepper type of chromatin and moderate amphophilic cytoplasm. The left thyroid nodule showed a pure plasmacytoid morphology with a round eccentric nucleus having a salt and pepper chromatin. Some of the cells showed multinucleation with intra-nuclear inclusions. The cells had abundant amphophilic cytoplasm. Though these tumors are known to show a mixed plasmacytoid and spindle phenotype in some instances, a pure plasmacytoid morphology one side and spindle cell on the other is unusual. The metastatic deposit in the right cervical lymph node showed plasmacytoid morphology though the right thyroid nodule shows a pure spindle cell morphology. This morphologic variation was confirmed on the histological examination of these nodules, which also demonstrated amyloid deposition. The morphologic variation is interesting in context of MEN syndrome. It is possible that the multicentric tumors associated with familial syndromes are more often demonstrate morphologic heterogeneity. This feature though not very clinically significant but may be important to consider while making a cytological diagnosis. This finding is also interesting given the genotype and cytomorphological correlation reported in some papers. The tumors arising in families carrying the same mutation have been reported to show the same morphology and specific morphologic subtypes have been associated with specific mutations. Codon 918 mutation is found to be associated with small/round and spindled cells and codon 634 with small/round, large oval to polygonal forms. The results of the genetic testing revealed a RET gene mutation C620R (TGC>CGC), consistent with MEN2A syndrome. In contrast to this case, Chang et al have not seen spindle cell morphology in a family with codon 620 mutations. However, the variation in the morphology of the two sides could represent a presence of additional mutations along with the germline mutation in RET oncogene or morphologic heterogeneity with codon 620 mutations. Additional studies are required to in this area to establish whether a genotype-phenotype relationship exists for medullary thyroid carcinomas.
Answer Key:
- A, Medullary carcinoma, spindle type morphology (right side) plasmacytoid morphology (left side)
- B, MEN2A
- C, RET
- B, Calcitonin
- C, C-cell hyperplasia
REFERENCES
DeLellis. Tumors of the thyroid gland (C-cells) In Khan A. (Eds) Surgical Pathology of Endocrine and Neuroendocrine tumors. Humana Press, 2009, p83-98
DeLellis RA. Medullary Thyroid carcinoma In Hunt J L (Eds) Molecular Pathology of Endocrine Diseases. Springer P 103-121
Khan A and Nose V. Pathology of Thyroid gland. In Lloyd RV (Eds) Endocrine Pathology: Differential Diagnosis and Molecular Advances. Springer. P 181- 235
Pitman M B, Oretel Y C, Gresinger K. Medullary thyroid carcinoma. In Syed Z. Ali and Edmund S. Cibas (eds.), The Bethesda System for Reporting Thyroid Cytopathology. Springer. P 117-127
Matias-Guiu X , De Lellis R . Medullary thyroid carcinoma: a 25-year perspective. Endocr Pathol. 2014 Mar;25(1):21-9.
Wells SA Jr , Pacini F , Robinson BG , Santoro M . Multiple endocrine neoplasia type 2 and familial medullary thyroid carcinoma: an update. J Clin Endocrinol Metab. 2013 Aug; 98(8): 3149-64.
Pusztaszeri MP , Bongiovanni M , Faquin WC . Update on the cytologic and morphologic features of Medullary Thyroid carcinoma. Adv Anat Pathol. 2014 Jan;21(1):26-35
Chang JS , Chang CF , Yang WS , Chang TC . The relationship of cytomorphology of medullary thyroid carcinomas between family members with the same RET proto-oncogene mutation. Acta Cytol. 2011;55(6):556-62.
Chang TC , Wu SL , Hsiao YL . Medullary thyroid carcinoma: pitfalls in diagnosis by fine needle aspiration cytology and relationship of cytomorphology to RET proto-oncogene mutations. Acta Cytol. 2005 Sep-Oct; 49(5): 477-82.\
Frank-Raue K, Döhring J, Scheumann G et al. New mutations in the RET protooncogene-L881V – associated with medullary thyroid carcinoma and -R770Q – in a patient with mixed medullar/ follicular thyroid tumor. Exp Clin Endocrinol Diabetes 2010; 118: 550–553
Kumar P, Hodjati H, Monabati A et al. Medullary Thyroid Carcinoma, Rare Cytologic Findings. Acta Cytol. 2000 Mar-Apr; 44 (2), 181-4
Matias-Guiu X, DeLellis R, Moley JF et al. Medullary thyroid carcinoma. In: DeLellis RA, Lloyd RV, Heitz PU, eds. World Health Organization Classification of Tumors Pathology and Genetics of Endocrine Organs. Lyon: IARC Press, 2004, p86-91.
Contributed by:
Monica Vyas, MD
Malini Harigopal, MD
Xiu Sun, MD.
Susan Fernandez, MD
Renu Virk, MD
Department of Pathology
Yale University School of Medicine