Case of the Month ...
Clinical History:
A sixty year old man with a past history of prostate cancer, presented with hematuria. As a part of the hematuria work-up, a CT and MRI was performed which showed a 2.6 x 2.7 cm nodal mass. On EGD/EUS, this 3 cm mass was hypoechoic, solid and mildly heterogeneous with smooth, well defined borders and was located between the left lobe of the liver and pancreas. A EUS-FNA of the mass was performed. The clinical consideration was a metastatic prostate carcinoma.
Diagnosis & Discussion
click on image for larger version
Images:
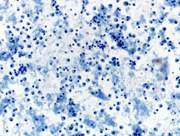
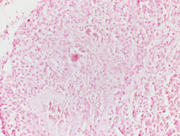
Figure: 1 Intraabdominal nodal mass FNA, Papanicolaou and Diff-quik Stains, 200 X; showing cellular smear comprising of plasmacytoid cells
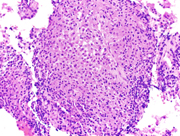
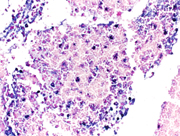
Figure 2. Cell block of FNA sample, 200X showing sheets of plasma/plasmacytoid cells.
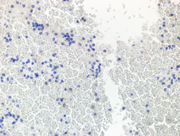
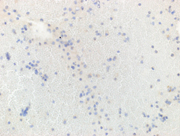
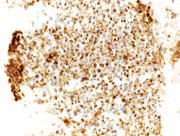
Figure 3. Immunohistochemical stains on the cell-block, 200X showing
3a. Plasmacytoid cells are negative for AE1/AE3 (cytoplasmic),
3b. Plamsacytoid cells negative for HMB 45, ruling out melanoma
3c & 3d: Plasma cells are negative for lambda light chain antibody, while positive for cytoplasmic kappa light chain antibody.
3e. Positive CD 138(cytoplasmic) in plasma cells,
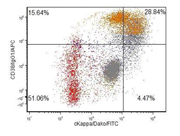
3f. Flow-cytometry report from Integrated Oncology revealed a clonal IgG kappa plasma cell population (~28%).
Questions:
1. The most likely diagnosis is:
a. Melanoma
b. Neuroendocrine tumor
c. Plasmablastic lymphoma
d. Plasma cell dyscrasias/ plasmacytoma e. lobular carcinoma of the breast2. On rapid on-site evaluation (ROSE), what would be the next crucial step in appropriately triaging the specimens so that the adequate work up can be performed?
a. Cell-block
b. Next Generation Sequencing
c. Flow-cytometry
d. Culture studies3. What are the characteristic cytomorphological features on the smears?
a. Single plasmacytoid small cells with salt and pepper chromatin, scant cytoplasm and small intracytoplasmic vacuoles.
b. Single plasmactyoid and groups of cells forming papillary groups and large intracytoplasmic vacuoles.
c. Dyscohesive plasmacytoid cells with eccentric nuclei, clumped chromatin, abundant basophilic cytoplasm and binucleated cells.
d. Single plasmacytoid cells with prominent macro-nucleoli, binucleate cells, intranulcear inclusions and vesicular chromatin.4. What is the required panel of immunohistochemical stains for reaching the diagnosis?
a. CD 20, CD 3, Kappa & Lambda
b. AE1/AE3, Synaptophysin and Chromogranin
c. AE1/AE3, HMB45, Melan A
d. CD 138, Kappa & Lambda e. CD 163, CD 34, Kappa5. Once the final diagnosis is made, what are the next important steps on the management perspective?
a. Whole body imaging with CT/PET study
b. SPEP/UPEP
c. Bone marrow biopsy with cytogenetic and FISH study
d. All of the aboveDiscussion:
Extramedullary presentation of multiple myeloma (MM) is a rare presentation, occurring in 10 % – 15 % of patients (2). Five percent of patients with multiple myelomas are diagnosed with extramedullary plasmacytomas, and even less than that are diagnosed as a primary lesion (3). The diagnosis of extramedullary plasmacytoma without the clinical signs or symptoms in an unknown primary is difficult and challenging. This case highlights a first presentation of multiple myeloma diagnosed by EUS-FNA and presented as an unknown primary in the setting of known history of prostate carcinoma; and the diagnosis had a major impact on the treatment and management.
EUS-FNA is a minimally invasive and effective method for sampling of malignant and benign lesions of the pancreas, the biliary system, the gastrointestinal tract wall and of lymph nodes in its vicinity playing a key role in primary diagnosis and in staging of gastrointestinal malignant tumors(6). The diagnosis of extramedullary plasma cell neoplasm by imaging studies is difficult due to lack of typical features on CT, magnetic resonance imaging (MRI) and ultrasound (12).
In current case, the Diff-Quik and Papanicolaou stained slides showed highly cellular smear consisting of numerous, dyscohesive, single, large and small plasmacytoid cells, with clumped chromatin and abundant basophilic cytoplasm. Some cells were binucleated. Based on this morphology, the differential diagnoses included plasmablastic lymphoma, plasmacytoma, melanoma, neuroendocrine tumor and lobular carcinoma of the breast (17, 18). Hence, an aliquot of the sample was submitted for flow-cytometry with a request to add plasma cell markers; and part of the specimen was collected for cell-block for ancillary studies. Flow-cytometry was negative for lymphoma, however showed that ~28% of the cells were CD 38 positive with kappa light chain restriction.On immunohistochemical studies, these plasmacytoid cells were positive for CD79a, CD138 and kappa light chain while lambda light chain was negative establishing its monoclonality. The plasmacytoid cells were negative for pan-cytokeratin, HMB-45 and synaptophysin and chromogranin antibodies. Once the diagnosis of extramedullary plasma cell dyscrasia was made on cytomorphology and immunophenotype; the cytopathologist recommended serum and urine protein electrophoresis, whole body imaging (CT/PET), 24 hour urine protein analysis and bone marrow biopsy with cytogenetic and FISH study in the report. This step is crucial to rule out underlying multiple myeloma. Based on the cytomorphological diagnosis and recommendation, a CT/PET scan was requested, which revealed multiple FDG avid lesions, including extensive bone lesions, markedly elevated kappa free light chain and kappa: lambda ratio. The case was finally diagnosed as light chain restricted multiple myeloma.
Above mentioned steps in clinical management are important to rule out presence of underlying multiple myeloma as multiple myeloma complicated by secondary extramedullary plasmacytoma carries a poor prognosis, like in current case.
In summary, we present herein a rare presentation of multiple myeloma as an extramedullary plasmacytoma of an unknown solitary, intraabdominal mass. We also highlight herein, the importance of EUS-FNA as a minimally invasive, cost effective diagnostic modality with very low rate (1.5 to 2%) of complications and high diagnostic yield (6, 16). This case emphasizes the importance of appropriately triaging the specimen during the rapid on-site evaluation, obtaining the material for ancillary studies to aid to the appropriate diagnosis and, avoiding diagnostic pitfalls. The role of cytopathologist during on-site evaluation remains crucial and pivotal for the successful diagnosis. Awareness of the unusual presentation of this entity and the need for high degree of suspicion is required to avoid delay in diagnosis, and for appropriate treatment.
Answers:
1: d
2. c
3. c
4. d
5. d
References:
1. J Gastrointest Oncol. 2015 Apr; 6(2):E7-9. doi: 10.3978/j.issn.2078-6891.2014.092. Extramedullary plasmacytoma of the gallbladder diagnosed by endoscopic ultrasound fine needle aspiration (EUS-FNA). St Romain P1, Desai S1, Bean S1, Jiang X1, Burbridge RA1.
2. JOP. 2012 Jan 10; 13(1):26-9. Use of endoscopic ultrasound in diagnosing plasmacytoma of the pancreas. Miljkovic' M (1), Senadhi V.
3. Endosc Ultrasound. 2016 Mar-Apr; 5(2):134-6. doi: 10.4103/2303-9027.180481. Endoscopic ultrasound-fine needle aspiration: A novel way to diagnose a solitary extramedullary plasmacytoma of the liver. Husney J(1), Guttmann S, Anyadike N, Mayer I, Rahmani R.
4. Endoscopy. 2010; 42 Suppl 2:E263-4. doi: 10.1055/s-0030-1255659. Epub 2010 Oct 7. Pancreatic head mass of unusual etiology: multiple myeloma diagnosed by endoscopic ultrasound-guided fine needle aspiration. Pinto-Marques P1, Martins C, Mendonça E, Castro H, Serra D.
5. Rinsho Ketsueki. 2009 Feb; 50(2):102-6. [Hepatic hilus extramedullary plasmacytoma diagnosed by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) biopsy in a relapsed multiple myeloma]. [Article in Japanese] Oyama N1, Ise M, Mimura N, Tsujimura H, Sakai C, Kumagai K.
6. Best Pract Res Clin Gastroenterol. 2009; 23(5):743-59. doi: 10.1016/j.bpg.2009.05.006. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology - An overview. Jenssen C1, Dietrich CF.
7. Diagn Cytopathol. 2015 Apr; 43(4):278-86. doi: 10.1002/dc.23207. Epub 2014 Aug 4. Onsite cytopathology evaluation and ancillary studies beneficial in EUS-FNA of pancreatic, mediastinal, intra-abdominal, and submucosal lesions. Mehmood S1, Jahan A, Loya A, Yusuf MA.
8. Hiller N et al. Plasmacytoma of pancreas. Isr Med Assoc J 2004;6(11):704-5
9. Shizuhiro H et al. Hepatobillary Pancreat Surg 2002;9:111-115
10. Varettoni M et al. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma:A longitudinal study on 1003 consecutive patients.Ann Oncol 2010;21:325-330
11. Wirk B, Wingard JR, Moreb JS. Extramedullary disease in plasma cell myeloma: the iceberg phenomenon. Bone Marrow Transplant 2013; 48:10-8. [PubMed]
12. Hammond NA et al. Imaging features of the less common pancreatic masses.Abdom Imaging 2013;38:561-572
13. Mitchell DG et al. Obstructive jaundice due to multiple myeloma of the pancreatic head: CT evaluation. J Comput Assist Tomogr. 1985, 9: 1118-1119.
14. Kazama T et al. Multiphasic CT and MRI appearances of extramedullary multiple myeloma involving the stomach, pancreas, and bladder. Clin Imaging. 2005, 29: 263-265.
15. Hawes RH. Indications for EUS-directed FNA. Endoscopy 1998; 30(1):A155-7.
16. Yoshinaga S et al. Role of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) for diagnosis of solid pancreatic masses. Dig Endosc 2011; 23(1):29-33.
17. Endosonography 3rd edition, Chapter 23: Cytology primer for endosonographers, Jhala algorithm p310-330; expert consult.
18. Jhala D, Eloubeidi M, Chhieng DC, et al. Fine needle aspiration biopsy of the islet cell tumor of pancreas: a comparison between computerized axial tomography and endoscopic ultrasound-guided fine needle aspiration biopsy. Ann Diagn Pathol. 2002;6:106–112.
Contributed by:
Sharvari Dalal M.D., FCAP; FASCP Director Cytopathology & Clinical Chemistry CMC Philadelphia VA Medical Center
Darshana Jhala M.D., B.Mus., FCAP Professor of pathology University of Pennsylvania Perelman School of Medicine Chief, Pathology and Laboratory Medicine CMC Philadelphia VA Medical Center