Case of the Month ...
Clinical History:
A 44 year old female with a history of infertility, status post embryo transfer and molar pregnancy, was found to have a 3.6 cm left renal mass 1 month after suction dilation and curettage for a complete mole. An ultrasound guided fine-needle aspiration biopsy was performed.
Diagnosis & Discussion
click on image for larger version
Images 1-4g:
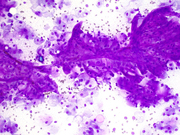
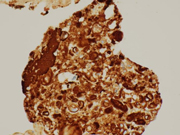
Figure 1. FNA direct smear of kidney mass, Diff Quik stain, 200x
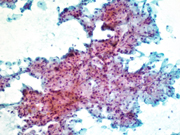
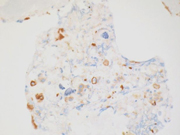
Figure 2. FNA direct smear of kidney mass, Papanicolaou stain, 200x
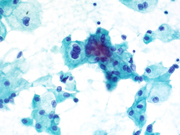
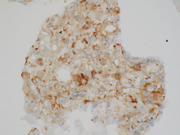
Figure 3. FNA direct smear of kidney mass, Papanicolaou stain, 600x
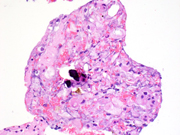
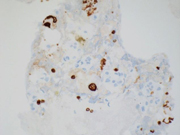
Figure 4A-F. Cell block, 400x, H&E stain (A), CK7 (B), AE1/AE3 (C), CD117 (D), Vimentin (E), Carbonic Anhydrase IX (F), HMB45 (G)Questions:
- What is the diagnosis?
- Gestational trophoblastic tumor
- Papillary renal cell carcinoma
- Chromophobe renal cell carcinoma
- Oncocytoma
- Clear cell renal cell carcinoma
- Which cytologic feature characterizes this tumor?
- Necrosis
- True fibrovascular cores
- Abundant granular cytoplasm
- Prominent nucleoli
- Which of the following is true most often of this lesion?
- Ill-defined lesion radiologically
- Central stellate scar
- Perinephric invasion
- Fat containing lesion
- Which immunoprofile is most likely to occur in this entity?
- CD117+, CK7+, Vimentin-, Carbonic Anhydrase-
- CD117+, CK7-, Vimentin-, Carbonic Anhydrase-
- CD117-, CK7-, Vimentin+, Carbonic Anhydrase+
- CD117-, CK7+, Vimentin+, Carbonic Anhydrase+
Discussion:
Chromophobe renal cell carcinomas (ChRCC) are uncommon and compose 5-7% of renal cell carcinomas. ( 1 ) They occur most commonly sporadically, in all ages with a peak incidence in the sixth decade. ( 1 ) ChRCC has a 5 and 10 year survival rate of 100% and 90% respectively. ( 2 ) These tumors are thought to arise from the intercalated cells of the cortical segment of the collecting duct. ( 3 )
Radiologically, ChRCC are well-circumscribed and homogeneous. Cystic change, necrosis, perinephric invasion and vascular involvement are rare. Often there is a central stellate scar and spoke-wheel enhancement. These findings however, are also common to oncocytomas. ChRCCs rarely contain fat and more commonly contain calcifications as compared to clear cell RCC- a feature shared with papillary RCC. ChRCCs display intermediate vascularity as compared to hypervascularity seen in clear cell RCC and hypovascularity seen in papillary RCC. ( 4 )
Cytomorphologically, fine-needle aspiration (FNA) smears are cellular, comprised of cells with abundant granular cytoplasm which are somewhat less cohesive than clear cell RCC. They display “koilocytoid” appearance with anisonucleosis, binucleation, hyperchromasia, irregular nuclear contours, grooves and occasional pseudoinclusions and calcifications. ( 5 )
Grossly, ChRCCs are well circumscribed, unencapsulated large tumors with a mean diameter of 7 cm. They display light tan to brown color, often with a central scar. ChRCCs are generally confined to the kidney. ( 1 )
Histologically, ChRCCs display a nesting or alveolar arrangement with occasional microcystic and adenomatous growth patterns. The cells display abundant pale acidophilic cytoplasm with sharply defined cell borders and perinuclear clearing, due to the numerous microvesicles which can be seen on electron microscopy. Hales colloidal iron can be used to stain the vesicles which would indicate the presence of acidic mucins. ( 6 )
Immunohistochemically, ChRCCs are typically positive for several stains that also show immunopositivity in oncocytomas including: E-cadherin, Parvalbumin, Claudins 7 and 8, CD117, CK18, AE1/AE3 (infrequently) and Pax8 (expressed in almost all RCCs). Additional stains expressed by ChRCC, but not commonly expressed by oncocytomas include: CD82, and CK7. ( 7 )
ChRCCs are negative for Vimentin (expressed in clear cell and papillary RCC), alpha-Methylacyl coenzyme A racemase (AMACR) (expressed in papillary RCC and 68% of clear cell RCCs), carbonic anhydrase IX (expressed in clear cell and collecting duct RCC, urothelial carcinoma, and carcinomas of the endometrium, stomach, cervix, breast, lung, and liver, and in mesotheliomas, brain and neuroendocrine tumors), and S100A1 (expressed in oncocytomas, which can be another key immunostain to distinguish ChRCC from oncocytomas). ( 7 )
Several chromosome losses are common to ChRCC and include loss of Y, 1, 2, 6, 10, 13, 17 and 21. Mictochondrial DNA somatic mutations are common as well. 27-32% of cases display TP53 mutation, 9% display PTEN mutations and 12% display TERT rearrangements. ( 1 )
The differential diagnosis for ChRCC includes Oncocytomas, clear cell RCC and papillary RCC (type 2). ChRCCs show more irregular nuclear contours and nuclear size variation compared to oncocytomas. Histologically, oncocytomas display cells in rounded, discrete nested arrangements as compared to ChRCCs which display continuous trabeculae. Oncocytomas often display more stroma between cell nests as compared to the closely packed trabeculae of ChRCC with little intervening stroma. Hale's colloidal iron is picked up by the cells of ChRCC but rarely by the cells of oncocytoma. ( 5 ) Immuonstaining for CK7 can be the most helpful in distinguishing the two entities in order to achieve diagnostic confirmation of ChRCC, especially at the time of FNA, prior to analysis of the histological architecture. ( 7 ) ChRCCs also have increased anisocytosis and anisonucleosis as well as nuclear contour irregularity as compared to clear cell RCCs. Additionally, ChRCCs are more hyperchromatic and lack the nucleoli of clear cell RCC. ( 5 ) The two can be distinguished using immunohistochemistry, with CK7, CD117 and E-cadherin commonly expressed in ChRCC (and rarely in clear cell RCC), and Carbonic Anhydrase IX and Vimentin commonly negative in ChRCC but positive in clear cell RCC. ( 7 ) Papillary RCC (type 2) can be distinguished from ChRCC by its large grade 3 nucleoli and presence of true papillae. ( 5 ) While papillary RCC is similar to ChRCC immunohistochemically with the expression of CK7, ChRCC can be distinguished from papillary RCC by analyzing an immunohistochemical panel including CD117 and E-cadherin (positive in ChRCC) and Vimentin and AMACR (positive in papillary RCC). ( 7 )
Uncommonly, ChRCC may be familial can occur in association with Birt-Hogg-Dube syndrome, an autosomal dominant condition characterized by cutaneous fibrofolliculomas, pulmonary cysts and multifocal or bilateral renal tumors. Often they display ChRCCs with hybrid features of oncocytomas. This syndrome is linked to BHD/FLCN gene mutation on chromosome 17p11.2 encoding folliculin, a tumor suppressor protein downstream effectors of AMPK and mTOR pathways. ( 3 )
Answer Key:
- C
- C
- B
- A
6 Rosai J: Rosai and Ackerman's Surgical Pathology, ed 10. Mosby, 2011.
Contributed by: Tamar C. Brandler MD, MS and Dr. Joan Cangiarella MD
New York University Langone Medical Center