Case of the Month ...
Clinical History:
A 61 year old white male presented with abdominal pain and early satiety. He denied chest pain, reflux, or shortness of breath. Physical exam revealed no palpable abdominal masses. As part of the workup, esophagogastroduodenoscopy was performed, revealing a gastric sub-mucosal mass at the antrum measuring 3.5 cm. The mass was arising from muscularis propria and had two lobules on ultrasound examination. Endoscopic ultrasound-guided FNA was performed.
Diagnosis & Discussion
click on image for larger version
Figure 4 Figure 5 Figure 6 Figure 7 Figure 8 Images 1-8:
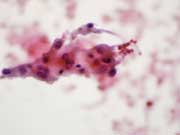
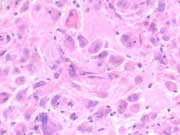
Figure 1: Endoscopic ultrasound-guided FNA. Pap stained smear, high magnification.
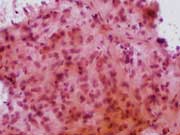
Figure 2: Endoscopic ultrasound-guided FNA. Pap stained smear, high magnification.
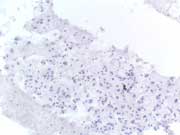
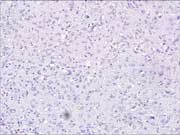
Figure 3: Endoscopic ultrasound-guided FNA. Cell Block, CD117 IHC stain, high magnification.
Figure 4: Endoscopic ultrasound-guided FNA. Cell Block, DOG-1 IHC stain, high magnification.
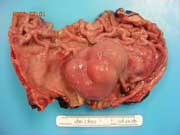
Figure 5: Partial gastrectomy specimen, gross examination.
Figure 6: Partial gastrectomy specimen. H&E stain, high magnification.
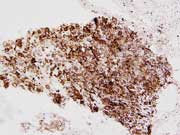
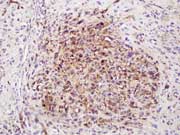
Figure 7: Partial gastrectomy specimen. CD-117 IHC stain, medium magnification.
Figure 8: Partial gastrectomy specimen. DOG-1 IHC stain, medium magnification.Figure 1: Loosely cohesive group of epithelioid cells with round nuclei and moderately abundant eosinophilic cytoplasm with ill-defined borders and traversing capillary is noted in the background. Endoscopic ultrasound-guided FNA. Pap stained smear, high magnification.
Figure 2: Epithelioid neoplastic cells with prominent intranuclear inclusions and binucleation. Endoscopic ultrasound-guided FNA. Pap stained smear, high magnification.
Figure 3: CD-117 shows negative staining. Endoscopic ultrasound-guided FNA. Cell Block, CD117 IHC stain, high magnification.
Figure 4: DOG-1 shows positive staining. Endoscopic ultrasound-guided FNA. Cell Block, DOG-1 IHC stain, high magnification.
Figure 5: Partial gastrectomy gross specimen showing submucosal mass. Mucosa is intact.
Figure 6: Tumor is composed of epithelioid cells with vesicular round nuclei and abundant eosinophilic cytoplasm. Binucleation is noted. H&E stain, high magnification.
Figure 7: CD-117 shows negative staining. Partial gastrectomy specimen. CD-117 IHC stain, medium magnification.
Figure 8: DOG-1 shows positive staining. Partial gastrectomy specimen. DOG-1 IHC stain, medium magnification.Questions:
1. What is the diagnosis:
A. Leiomayoma
B. Schwannoma
C. Gastrointestinal stromal tumor
D. Hemangioma2. What is the most common mesenchymal tumor of GI tract:
A. Leiomayoma
B. Schwannoma
C. Gastrointestinal stromal tumor
D. Hemangioma3. Which of the following might aid in confirmation of this neoplasm?
A. S-100(+), vimentin(+), Mart-1(+), DOG-1(-)
B. SMA (+), desmin(+), CD-117(-), DOG-1 (-)
C. CD34 (+), SMA (-), CD-117(-), DOG-1(+)
D. CD-117(-), synaptophysin(+), chromogranine(+), DOG-1(-)4. What cell type is most commonly seen in this neoplasm?
A. Epithelioid cell
B. Spindle cell
C. Signet ring cell
D. Multinucleated cell5. Which gene mutation is most commonly seen in this neoplasm?
A.PDGFRA
B. KRAS
C. KIT
D.LKB6. Gastrointestinal stromal tumors (GISTs) are always c-kit (CD-117) positive:
A. True
B. FalseDiagnosis and Discussion:
Cytopathologic Findings:
Six alcohol fixed slides and 30 cc of cytolyte with the aspirated specimen were sent to the cytology lab, from which Papanicolau-stained smears, one cell block, and one liquid-based slide (Thin Prep) were prepared. Papanicolaou-stained smears showed a cellular sample composed of loosely cohesive groups of cells. The cells were predominantly epithelioid with eccentric oval to round shaped nuclei, small or inconspicuous nucleoli, and moderate to abundant eosinophilic or amphophilic granular cytoplasm with ill-defined borders (Picture 1). . Prominent intranuclear pseudoinclusions and binucleation were present (Picture 2). The cell block preparation was moderately cellular. Cells were arranged in sheets and showed cytologic features similar to smears.
An immunocytochemical panel was performed with the following results: negative for TTF-1, CK7, CK20, and A103; and weakly positive for S100. Initially, the case was interpreted as a poorly differentiated large cell malignancy. Melanoma was still in the differential diagnosis due to the prominent intranuclear inclusions, epithelioid morphology and faint S100 positivity. Additional immunocytochemical stains were performed and the results were as follows: CD117 negative (Picture 3), CD34 positive and smooth muscle actin (SMA) focally positive, and leukocyte common antigen (LCA) negative. Based on these stains, an amended diagnosis was then issued stating that the tumor was most consistent with a GIST and that a tissue sample was needed to determine biologic behavior based on histologic criteria.
Histopathologic Correlation:
A partial gastrectomy was performed at a later date. The specimen measured 7 x 6 x 4 cm and showed a submucosal neoplasm (Picture 5). Sectioning revealed a 3.5 x 3.2 x 3.0 cm multilobulated well-circumscribed tan-gray tumor confined to the wall of the stomach. A second smaller tumor nodule of 1.5 cm was seen near the proximal margin of resection.
Sections of tumor stained with hematoxylin and eosin showed a neoplasm composed predominantly of large epithelioid cells with pleomorphic nuclei, prominent small nucleoli and abundant eosinophilic to clear cytoplasm (Picture 6). Binucleation and multinucleation were noted. Occasional cytoplasmic vacuoles and intranuclear inclusions were present.
Immunohistochemical stains were as follows: CD117 negative (Picture 7), CK, Vimentin, EMA, CD-31, Mart-1, HMB-45 and S-100 negative; CD-34 positive and SMA weakly positive. The case was signed out as a multifocal epithelioid GIST. Based on size (3.5 cm) and mitotic activity (none in fifty 400X fields) the tumor was determined to be low risk for biologically aggressive behavior.
DOG-1, a newer immunohistochemical marker for GIST, was not available in our laboratory at the time of diagnosis. Retrospectively, DOG-1 immunohistochemistry was performed on both the cytology cell block and the tissue block for histology, and was found to be strongly positive in neoplastic cells (Pictures 4 and 8).
Discussion:
GISTs are the most common gastrointestinal mesenchymal tumors, occurring most frequently in the stomach (about 70%), though they may occur anywhere in the gastrointestinal tract (1, 2, 3). These tumors are thought to be derived from intestinal cells of Cajal, which are intestinal pacemaker cells involved in peristalsis (1, 4, 5). GISTs are usually found to have a mutation of CD-117 (KIT), a defining feature of this tumor type, and an early step in the pathogenesis 4. KIT is a transmembrane tyrosine kinase receptor normally expressed by the intestinal cells of Cajal and that is constitutively activated by the mutation leading to increased cell survival and proliferation (4).
GISTs usually consist of one of two main cells types, spindle or epithelioid. Rarely, both cell patterns may be seen in the same tumor (2). The epithelioid variant is less common, occurring in approximately 20-30% of GISTs (1). Among the epithelioid type, several variants have been noted including sclerosing, discohesive, hypercellular, and sarcomatous (2). In epithelioid GISTs, cytologic smears are usually cellular with the cells found singly or in loose clusters. On Papanicolaou stained slides, the epithelioid cells contain moderate amounts of granular or clear amphophilic to eosinophilic cytoplasm. Nuclei are round and often binucleated with occasional intranuclear inclusions. Other common findings include traversing vessels, irregular nuclear contour, and small nucleoli (1).
KIT (CD117) is the characteristic immunohistochemical marker of GIST, found in more than 90% of cases. Staining is usually strong and widespread and may be distributed diffusely in the cytoplasm, localized to a perinuclear dot, or membranous (2). Most GISTs (about 80%) also express CD34, and smooth muscle actin is demonstrable in about 30%. Some of these CD117 negative tumors contain KIT mutations detected by molecular tests, while others contain PDGFRA mutations. KIT negative GISTs with PDGFRA mutations are more likely to show epithelioid features, whereas KIT positive tumors are usually of the spindle cell type. Another factor to consider is that immunoreactivity may change after targeted therapy with imatinib mesylate, as loss of CD117 and CD34 immunopositivity has been reported.
DOG-1 (discovered on GIST 1), a calcium-dependent chloride channel is a sensitive and specific immunohistochemical marker for in GISTs (7). In a large histologic study (8), sensitivity was comparable to that of KIT, but in a study of 1,168 GISTs for histology and 672 other tumor types, DOG-1 was found to be 94.4% sensitive for GIST, compared to 94.7% sensitivity with KIT. However, DOG-1 was a more sensitive marker in epithelioid GISTs. It was also highly specific for GIST, and as it was negative for many tumors that are potentially KIT-positive such as melanoma, seminoma, Ewing sarcoma, and extramedullary myeloid tumors. In a smaller FNA study, DOG-1 was found to be 100% sensitive and 100% specific for GIST, and all KIT-negative GISTs (nine of thirty) were positive for DOG-1(7). DOG-1, therefore, is a useful marker for GIST, particularly in KIT-negative cases (8).
The most powerful prognostic factors are tumor size and mitotic activity ( 9). Tumors ≥ 5 cm and with ≥ 5 mitosis per 50 HPFs have a poorer prognosis. Patients with tumors ≤ 2 cm and ≤ 5 mitoses per HPF have essentially no chance of progressive disease, whereas those with tumors between 5-10 cm and ≥ 5 mitoses per 50 HPFs have a 55% chance of progressive disease (10). Anatomic location is an independent prognostic factor; tumors in the rectum or small intestine have the worst prognosis, while those from the esophagus or stomach have a relatively more favorable outcome (3).
GISTs usually respond to imatinib mesylate (Gleevec), a tyrosine kinase inhibitor originally designed to be used in cases of chronic myelogenous leukemia (1). Gleevec has also shown promise as a single agent in metastatic GIST (4) and even often produces a clinical response in cases that are KIT negative; these tumors may have KIT gene mutations not detected by immunohistochemistry. Gleevec may be lifesaving for some patients (8).
Clinicians should be aware that traditional endoscopic biopsies are usually insufficient for the diagnosis of GIST as they are too superficial. GISTs are deeper mesenchymal tumors commonly arising from the muscularis mucosa or muscularis propria. They are, however, quite amenable to diagnosis by fine needle aspiration via endoscopic ultrasonography (EUS). Immunocytochemical analysis can be performed on aspirates for definite diagnosis (3). In fact, EUS alone provides morphologic information with defined features that suggest malignancy, including size, margins, echogenic foci or cystic spaces, and growth rate in follow-up EUS (9). In a study of 53 consecutive EUS-FNAs of the GI tract, this sampling method along with immunohistochemical analysis was found to be accurate for the diagnosis of GIST. In 29 surgically resected cases, EUS-FNA with immunohistochemistry had a sensitivity of 100%, specificity of 80%, positive predictive value of 96%, and negative predictive value of 100%( 9).
The differential diagnosis of epithelioid GIST is vast and includes neuroendocrine tumors, hepatocellular carcinomas, melanomas, large cell carcinomas, and other neoplasms with epithelioid differentiation (1). The distinction between GIST and from endocrine tumors may be challenging because of the plasmacytoid appearance of the cells along with round nuclei and finely granular cytoplasm. Immunocytochemistry with positive chromogranin and synaptophysin in neuroendocrine tumors helps to confirm the diagnosis. GIST is also morphologically similar to melanoma because of Epithelioid GIST with dyshesive cells, large eccentric nuclei, prominent nucleoli, and intranuclear inclusions may resemble melanoma. However, melanoma markers are usually negative in GIST including S-100, Mart-1, and HMB-45. S-100 has been found to be positive in less than 1% of GISTs (2), although this can be troublesome as we describe in this case, however other melanoma markers are negative in GIST. GIST can also be difficult to differentiate from hepatocellular carcinoma, especially when traversing capillaries are seen (1), and particularly in tumors that are negative for CD117. Markers such as Hep Par 1 and alpha-fetoprotein are helpful in these cases (1).
In summary, cytopathologists should consider GIST in the differential diagnosis of an epithelioid neoplasm. A minority of GISTS are CD117 negative; in these cases DOG-1 may be diagnostically useful. An accurate GIST diagnosis is important because even in KIT negative tumors, patients may respond to targeted therapy.Answers:
1. C
2. C
3. C
4. B
5. C
6. BReferences:
1. Dong Q, McKee G, Pitman M, et al. Epithelioid variant of gastrointestinal stromal tumor: diagnosis by fine needle aspiration. Diagn Cytopathol. 2003;29:55-60.
2. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52-68.
3. Logrono R, Bhanot P, Chaya C, et al. Imaging, morphologic, and immunohistochemical correlation in gastrointestinal stromal tumors. Cancer Cytopathol. 2006;108:257-266.
4. Medeiros F, Corless, CL, Duensing A, et al. KIT-negative gastrointestinal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004;28:889-894.
5. Wang L, Vargas H, French SW. Cellular origin of gastrointestinal stromal tumors: a study of 27 cases. Arch Pathol Lab Med. 2000;124:1471-1475.
6. Pauwels P, Debiec-Rychter M, Stul M, et al. Changing phenotypes of gastrointestinal stromal tumours under imatinib mesylate treatment: a potential diagnostic pitfall. Histopathol. 2005;47:41-47
7. Fatima N, Cohen C, Siddiqui MT. DOG1 utility in diagnosing gastrointestinal stromal tumors on fine-needle aspiration. 2011; March 11 online ahead of print publication.
8. Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors. Am J Surg Pathol. 2009;33:1401-1408.
9. Akahoshi K, Sumida Y, Matsui N, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077-2082.10. Bosman FT, Carneiro F, Hruban RH, eds., et al. WHO Classification of Tumours of the Digestive System. 4th ed. Lyon, France: International Agency for Research on Cancer; 2010:74-76.
Contributed by:
Magda Esebua, MD
Clinical Associate Professor of Pathology
Director, Cytology Section
University of Missouri School of Medicine
Department of Pathology and Anatomical Sciences
One Hospital Drive, M263 Med Sci Building
Columbia, MO 65212
Phone: 573-884-8950
Fax: 573-884-4612