Case of the Month ...
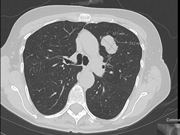
Clinical history: A 73-year-old female underwent an endobronchial, transtracheal ultrasound-guided fine needle aspiration (FNA) and biopsy of a 3.2 cm heterogenous well-circumscribed left upper lobe lung mass.
Authors
- Jing Wang and Claire W. Michael
Diagnosis & Discussion
click on image for larger version
Figure 3 Figure 4 Figure 5 Figure 6 Images 1-7:
- Figure 1: CT scan of lung mass
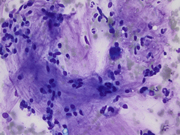
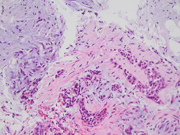
- Figures 2-3: FNA of lung mass (Diff-quick Stain 40x)
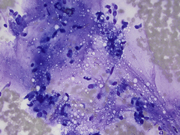
- Figure 4: FNA of lung mass (Pap smear 40x)
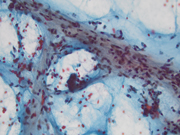
- Figure 5: FNA of lung mass (Cell block, H&E 4x)
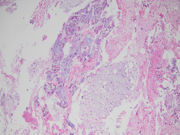
- Figures 6-7: Transbronchial biopsy of lung mass (H&E 20x,10x)
Questions:
- What is the best diagnosis based on the FNA and biopsy images?
- Pulmonary hamartoma
- Pleomorphic adenoma
- Pulmonary chondroma
- Chondrosarcoma, primary or metastatic
- Nondiagnostic sample with benign elements
- What is the molecular abnormality that supports the diagnosis?
- HMGA2-LPP translocation
- PLAG1 or HMGA2 rearrangements
- SDHA, SDHB or SDHC mutation
- HEY1-NCOA2 rearrangements
Answers:
Question 1: Correct answer is APulmonary hamartoma is a benign mesenchymal tumor composed primarily of varying degrees of mesenchymal tissue, including cartilage, fat, smooth muscle, and bone[1]. Typically, pulmonary hamartoma can be diagnosed through imaging, revealing a coin lesion with focal fat or “popcorn” calcification [2]. However, in the current case, the CT scan showed a well-circumscribed 3.2 cm mass without calcification for which hamartoma was suggested but further investigation to exclude malignancy was recommended. Smaller hamartomas lack internal fat or calcification, requiring follow-up CT scans or biopsy to exclude malignancy.
The FNA revealed numerous bronchial cells in a background of many fragments of loose chondroid matrix, myxoid material either loose or with interlacing fascicles, transgressing stroma and occasional vacuoles. The cell block showed similar findings. Furthermore, the transbronchial biopsy was comprised of cartilage, fibromyxoid tissue, adipocytes and invaginations of normal respiratory epithelium, consistent with pulmonary hamartoma. On FNA, these findings can be interpreted as non-diagnostic, mucinous bronchioloalveolar carcinoma or carcinoid [3]. However, the presence of a more rigidly structured myxoid matrix suggests hamartoma[4].
Pleomorphic in the lung typically manifests in the trachea and major bronchi. It consists of an admixture of epithelial and myoepithelial components along with chondroid stroma. However, it rarely contains mature cartilage and is devoid of entrapped bronchial epithelium [5]. Pulmonary chondroma consists entirely of benign cartilage without other mesenchymal components. Often, multiple lesions are associated with Carney triad, which consists of paragangliomas, gastrointestinal stromal tumors, and pulmonary chondromas [6]. Chondrosarcoma, characterized by cellular atypia, does not exhibit an admixture of mesenchymal tissue or entrapped bronchial epithelium. Metastatic chondrosarcoma usually manifests as multiple lung lesions [7].
Question 2: Correct answer is A
The high frequency of HMGA2-LPP translocation is associated with pulmonary hamartoma [8]. PLAG1 or HMGA2 rearrangements are commonly identified in pleomorphic adenoma [9]. Carney triad is associated with SDHA, SDHB, or SDHC mutation [10]. Mesenchymal chondrosarcoma is characterized by recurrent HEY1-NCOA2 rearrangements[11].
REFERENCES
- Lundeen, K.S., et al., Pulmonary Hamartoma, in StatPearls. 2023: Treasure Island (FL).
- Suut, S., et al., Pictorial essay of radiological features of benign intrathoracic masses. Ann Thorac Med, 2015. 10(4): p. 231-42.
- Saqi, A., et al., Incidence and cytological features of pulmonary hamartomas indeterminate on CT scan. Cytopathology, 2008. 19(3): p. 185-91.
- Umashankar, T., et al., Pulmonary hamartoma: Cytological study of a case and literature review. J Cytol, 2012. 29(4): p. 261-3.
- Moran, C.A., Primary salivary gland-type tumors of the lung. Semin Diagn Pathol, 1995. 12(2): p. 106-22.
- Rodriguez, F.J., et al., Pulmonary chondroma: a tumor associated with Carney triad and different from pulmonary hamartoma. Am J Surg Pathol, 2007. 31(12): p. 1844-53.
- Yasin, J.T., et al., Primary Pulmonary Chondrosarcoma: A Case Report and Literature Review. J Clin Imaging Sci, 2020. 10: p. 3.
- von Ahsen, I., P. Rogalla, and J. Bullerdiek, Expression patterns of the LPP-HMGA2 fusion transcript in pulmonary chondroid hamartomas with t(3;12)(q27 approximately 28;q14 approximately 15). Cancer Genet Cytogenet, 2005. 163(1): p. 68-70.
- Andreasen, S., et al., Recurrent rearrangements of the PLAG1 and HMGA2 genes in lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic adenoma. Acta Ophthalmol, 2018. 96(7): p. e768-e771.
- Stratakis, C.A. and J.A. Carney, The triad of paragangliomas, gastric stromal tumours and pulmonary chondromas (Carney triad), and the dyad of paragangliomas and gastric stromal sarcomas (Carney-Stratakis syndrome): molecular genetics and clinical implications. J Intern Med, 2009. 266(1): p. 43-52.
- Wang, L., et al., Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer, 2012. 51(2): p. 127-39.