Case of the Month ...
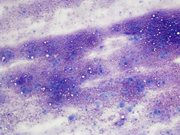
A 36-year-old with a firm left neck mass spanning level 1 and level 2 lymph nodes. A fine needle aspiration biopsy of the mass in the left submandibular area was performed.
Authors
- Heather Chen-Yost, MD, Cytopathology Fellow, University of Michigan
- Xin Jing, MD, Professor of Pathology, Cytopathology, University of Michigan
Diagnosis & Discussion
click on image for larger version
Figure 3 Figure 4 Figure 5 Figure 6 Figure 7 Figure 8 Images 1-8:
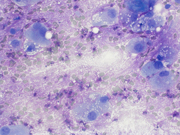
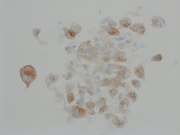
- Figure 1: Diff-Quik Stain, 100x
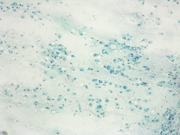
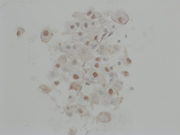
- Figure 2: Diff-Quik Stain, 400x
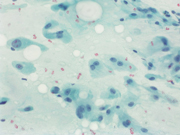
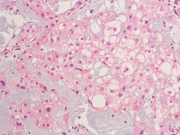
- Figure 3: Papanicolaou Stain, 100x
- Figure 4: Papanicolaou Stain, 400x
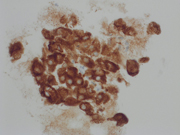
- Figure 5: Pan-cytokeratin (PanCK), 400x
- Figure 6: EMA, 400x
- Figure 7: S-100, 400x
- Figure 8: Mass resection, Hematoxylin and eosin stain, 200x
Additionally, the neoplastic cells were negative for SOX-10 and D2-40. Brachyury showed no convincing reactivity (images not shown).
Questions:
- Based on morphology and immunohistochemistry, which of the following is the best diagnosis?
- Metastatic chordoma
- Mypoepithelial carcinoma
- Squamous cell carcinoma
- Mucoepidermoid carcinoma
- Which of the following immunohistochemical markers is the most specific for this entity?
- EMA
- S100
- Brachyury d. INI-1 (loss)
Answers:
Question 1: Correct answer is A
The patient had a history of clival chordoma and was status post complete resection and radiation. A year later, he presented with a firm mass in the left submandibular area and reactive sialadenitis was favored clinically based on the radiation history.
The fine needle aspirate was highly cellular with atypical cells having dense and vacuolated cytoplasm. The cells were positive for epithelial markers (PanCK and EMA) and S-100. Brachyury was non-contributory.Morphologically, the differential included metastatic chordoma (based on the clinical history), squamous differentiation (based on the dense cytoplasm), mucoepidermoid carcinoma (based on the vacuolated cytoplasm and submandibular location) and myoepithelial carcinoma (based on the location and immunoprofile).
The clinical history, morphology and immunoprofile were characteristic of chordoma (corroborated on subsequent surgical resection).
Chordomas can be highly cellular 1 and have multi-vacuolated cytoplasm characteristic of physaliphorous cells (derived from the Greek word for bubble) 2. The nuclei tend to be monotonous and bland. The malignant cells can be binucleated. The background often shows metachromatic myxoid stroma.
Most commonly chordomas metastasize to the lungs, which occurs in about 50% of patients 3. However, one study reported metastatic clival chordoma to the salivary gland 4.Question 2: Correct answer is C
INI-1 loss is reported in poorly differentiated chordomas 10, which have increased nuclear atypia, increased mitotic figures, geographic necrosis and focal rhabdoid morphology. Additionally, physaliphorous cells and extracellular myxoid stroma are focal to absent.
Brachyury, a nuclear stain, is the most specific stain 4 with reported positivity in ~75-100% of chordomas 5.
S100 can also be positive in chondrosarcoma, which is a morphologic mimicker of chordoma. However, chondrosarcoma is negative for keratins and brachyury 6. Meanwhile myoepithelial tumors can express EMA, S100, and other cytokeratin markers but lack brachyury expression 7. Chordoma cells can mimic some cells in mucoepidermoid carcinomas, especially the mucous cells or those with clear cell changes. However, mucoepidermoid carcinomas express additional keratin markers aside from EMA such as CK5/6 and p63, and do not typically express S100 and brachyury 8,9.References:
- Young VA, Curtis KM, Temple HT, Eismont FJ, DeLaney TF, Hornicek FJ. Characteristics and Patterns of Metastatic Disease from Chordoma. Sarcoma. 2015;2015:517657.
- Gui X, Siddiqui NH, Guo M. Physaliphorous cells in chordoma. Arch Pathol Lab Med. 2004 Dec;128(12):1457-8. doi: 10.5858/2004-128-1457-PCIC.
- Wang H, Hoda RS, Faquin W, Rossi ED, Hotchandani N, Sun T, Pusztaszeri M, Bizzarro T, Bongiovanni M, Patel V, Jhala N, Fadda G, Gong Y. FNA biopsy of secondary nonlymphomatous malignancies in salivary glands: A multi-institutional study of 184 cases. Cancer Cytopathol. 2017 Feb;125(2):91-103.
- Vujovic S, Henderson S, Presneau N, et al Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006; 209 (2): 157– 165.
- Veronica Ulici, Jesse Hart; Chordoma: A Review and Differential Diagnosis. Arch Pathol Lab Med 1 March 2022; 146 (3): 386–395.
- Folpe AL, Agoff SN, Willis J, Weiss SW. Parachordoma is immunohistochemically and cytogenetically distinct from axial chordoma and extraskeletal myxoid chondrosarcoma. Am J Surg Pathol. 1999; 23 (9): 1059– 1067.
- Carter, C.S., Patel, R.M. Cutaneous soft tissue tumors: diagnostically disorienting epithelioid tumors that are not epithelial, and other perplexing mesenchymal lesions. Mod Pathol 33 (Suppl 1), 66–82 (2020).
- Azevedo RS, de Almeida OP, Kowalski LP, Pires FR. Comparative cytokeratin expression in the different cell types of salivary gland mucoepidermoid carcinoma. Head Neck Pathol. 2008 Dec;2(4):257-64.
- Sams RN, Gnepp DR. P63 expression can be used in differential diagnosis of salivary gland acinic cell and mucoepidermoid carcinomas. Head Neck Pathol. 2013 Mar;7(1):64-8.
- Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, eds. WHO Classification of Tumours of Soft Tissue and Bone. 5th ed. Lyon, France: International Agency for Research on Cancer; 2020. World Health Organization Classification of Tumours; vol 3.