Case of the Month ...
A 60-year-old woman with no significant medical history presented with episodes of abdominal pain and nausea. The CT scan revealed submucosal edema in the stomach body and antrum, a focal area of thinning in the body concerning for a peptic ulcer with segmental thickening at the hepatic flexure and surrounding enlarged mesenteric lymph nodes. Upper endoscopy showed reflux esophagitis without bleeding, and a large gastric ulcer which was biopsied. The histopathological examination of the biopsy revealed superficially eroded gastric mucosa with extensive intestinal metaplasia. Immunohistochemistry was negative for Helicobacter pylori.
Because of the persistent symptoms, she had repeat colonoscopy and upper endoscopy, which revealed a 40 x 21 mm hypoechoic mass in the antrum with sonographic evidence suggesting invasion into the serosa. This was staged T3 N2 by endosonographic criteria. There were also many malignant-appearing lymph nodes in the celiac region, the largest of which measured 16 mm, and endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) of a celiac lymph node was performed. (Figures 1 – 9)
Authors
- Haluk Kavus MD, Mohammed Amer Swid MD
- Sara Monaco MD, Geisinger Medical Center, Danville, PA
Diagnosis & Discussion
click on image for larger version
Figure 3 Figure 4 Figure 5 Figure 6 Figure 7 Figure 8 Figure 9 Images 1-9:
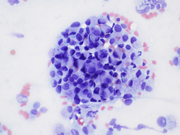
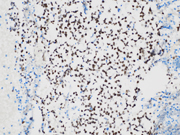
- Figure 1: EUS FNA of celiac lymph node, aspirate smear, Diff-Quik stained, 200x magnification.
- Figure 2: EUS FNA of celiac lymph node, aspirate smear, Diff-Quik stained, 400x magnification.
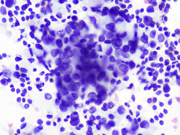
- Figure 3: EUS FNA of celiac lymph node, aspirate smear, Diff-Quik stained, 600x magnification.
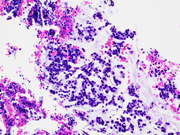
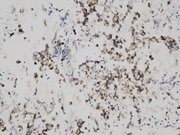
- Figure 4: EUS FNA of celiac lymph node, cell block, H&E stained, 200x magnification.
- Figure 5: EUS FNA of celiac lymph node, cell block, H&E stained, 200x magnification.
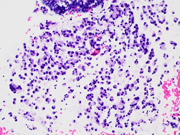
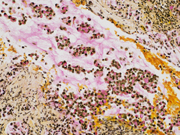
- Figure 6: EUS FNA of celiac lymph node, cell block, CDX2 immuno-stained, 200x magnification.
- Figure 7: EUS FNA of celiac lymph node, cell block, Synaptophysin immuno-stained, 200x magnification.
- Figure 8: EUS FNA of celiac lymph node, cell block, Mucicarmine stained, 200x magnification.
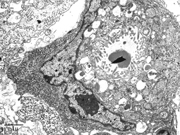
- Figure 9: Representative electron microscopic image of a signet-ring carcinoma cell.
Questions:
- Based on the clinical history, morphology, and immunohistochemistry, which of the following is the most appropriate diagnosis?
- Poorly differentiated neuroendocrine tumor
- Mixed adenocarcinoma (signet ring cell) and neuroendocrine carcinoma
- Signet ring adenocarcinoma
- Metastatic colon cancer
- Which special stain is likely to show positive results in signet-ring adenocarcinoma cells, distinguishing them from the neuroendocrine component with vacuolated cytoplasm in this case?
- Chromogranin stain
- Mucicarmine/PAS stain
- Synaptophysin stain
- INSM1 stain
- What electron microscopic feature distinguishes signet-ring carcinoma cells from vacuolated neuroendocrine cells?
- Presence of oval or round nuclei with a narrow-to-chunky border of chromatin in signet-ring cells
- Regular occurrence of endoplasmic reticulum, Golgi complex, and mitochondria in signet-ring cells
- Hyperchromatic nucleus peripherally displaced with microvilli and compressed by moderately electron-dense mucous granules in signet-ring cells
- The mucus granules are round or oval, and consisted of homogeneous, electron-dense areas tightly surrounded by a membrane
Answers:
Question 1: Correct answer is B
The microscopic analysis revealed two distinct cell types . The first type exhibited large cytoplasmic mucin vacuoles and eccentrically displaced nuclei (Figures 1, 2 and 5), leading to the diagnosis of signet-ring cell adenocarcinoma. The second type consisted of smaller, uniform neoplastic cells forming discrete structures, diagnosed as neuroendocrine carcinoma with few cells that had vacuolated cytoplasm, characterized by scant, eosinophilic granular cytoplasm and round nuclei with finely dispersed ("salt and pepper") chromatin (Figures 3 and 4).
Question 2: Correct answer is B
The signet ring adenocarcinoma had mucin vacuoles with intracellular mucicarmine (Figure 8) and PAS and were negative for neuroendocrine markers, helping to distinguish the 2 components. It is important to consider immunostains for both components (Figure 6 and 7) when one is identified, given that the neuroendocrine and signet ring components may be variably represented on smears or cell blocks from FNA specimens or be difficult to identify given the abundant cytoplasm or mild nuclear atypia.
Question 3: Correct answer is C
Electron microscopic examination revealed that the signet-ring carcinoma cells had a distinct ultrastructural pattern characterized by a hyperchromatic nucleus peripherally displaced and compressed by moderately electron-dense mucous granules. The cytoplasmic organelles in signet cells were mostly decreased and present at the periphery of the cell or between mucous granules. Intracellular lumens lined by microvilli were frequently observed. In contrast, the nuclei of the neuroendocrine cells were oval or round. A narrow-to-chunky border of chromatin along the nuclear periphery surrounded small nucleoli. Endoplasmic reticulum, Golgi complex, free ribosomes, mitochondria and lysosomes occurred quite regularly. These feature helped differentiate signet-ring carcinoma cells from neuroendocrine cells in the presented case.REFERENCES
- Bartley AN, Rashid A, Fournier KF, Abraham SC. Neuroendocrine and mucinous differentiation in signet ring cell carcinoma of the stomach: evidence for a common cell of origin in composite tumors. Hum Pathol. 2011;42(10):1420-1429.
- Zhang L, DeMay RM. Cytological features of mixed adenoneuroendocrine carcinoma of the ampulla: two case reports with review of literature. Diagn Cytopathol. 2014;42(12):1075-1084.
- Kaji K, Seishima J, Yamato M, et al. Clinical utility of endoscopic ultrasound-guided fine-needle aspiration in mixed adenoneuroendocrine carcinoma with signet-ring cells of the pancreas: a case report and review of the literature. Clin J Gastroenterol. 2016;9(1):43-48.
- Summer L Nugent, Steven C Cunningham,Borislav A Alexiev, Emily Bellavance, John C Papadimitriou, and Nader Hanna. Composite signet-ring cell/neuroendocrine carcinoma of the stomach with a metastatic neuroendocrine carcinoma component: a better prognosis entity. Diagn Pathol. 2007; 2: 43.
Table II
Classification and grading criteria for NENs of the gastrointestinal tract (1).
TerminologyDifferentiation
Grade
Mitotic ratea
Ki-67 indexb
NET, G1
Well-differentiated
Low
<2
<3%
NET, G2
Intermediate
2-20
3-20%
NET, G3
High
>20
>20%
SCNEC
Poorly differentiated
High
>20
>20%
LCNEC
>20
>20%
MiNEN
Well or poorly differentiated
Variable
Variable
Variable
Reference: The new WHO classification of gastrointestinal neuroendocrine tumors and immunohistochemical expression of somatostatin receptor 2 and 5
Oana Popa,1,2,* Sorina Maria Taban,1,* Stelian Pantea,3 Andrei Dorel Plopeanu,1,4 Robert Alexandru Barna,1,5 Marioara Cornianu,1 Anca-Ariana Pascu,5 and Alis Liliana Carmen Dema1,*
Exp Ther Med. 2021 Oct; 22(4): 1179.To differentiate between G3 and NEC :
Beside morphology, the molecular difference also help in differentating these two. Based on their molecular differences. Mutations in MEN1, DAXX, and ATRX are entity‐defining for well‐differentiated NETs, whereas NECs usually have TP53 or RB1 mutations
The microscopic evalatuion of the NECs divided intro small and large cells. The large cell NEC shows sheets of solid cells or trabeculae of large cells with eosinophilic cytoplasm, vesicular, pleomorphic nuclei with large nucleoli and the small cell NEC consist of small cells with scant cytoplasm and hyperchromatic nuclei , tumor necrosis and occasional desmoplastic stroma. Although it is quite challending to differentiate between well-differentiated G3 NETs and NEC, however, when you see areas of ares with monomorphic cells and organoid pattern of tumor cells, these feature are highly indication of this G3 NET.