Case of the Month ...
A 69 -year-old woman presented with abdominal pain. Computed Tomography (CT) scan detected a 4 cm periumbilical subcutaneous mass arising within the repaired hernia in abdominal wall, along with a 2.9 cm ill-defined nodular density in the left lower quadrant abdominal wall. In addition, the radiologist commented interval development of peritoneal nodularity/thickening and omental caking of unclear etiology, with a suspicion of peritoneal carcinomatosis. An ultrasound-guided FNA of the periumbilical subcutaneous mass was performed with cytology-assisted rapid onsite assessment, followed by a subsequent tissue biopsy.
Authors
- Xiaobing Jin, MD
- Judy Pang
- Xin Jing, MD
Diagnosis & Discussion
click on image for larger version
Figure 3 Figure 4 Figure 5 Figure 6 Figure 7 Figure 8 Figure 9 Images 1-9:
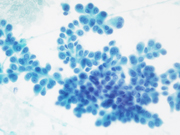
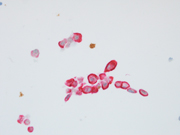
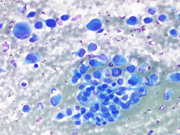
Figure 1: conventional smear, Diff Quik stained, 400x
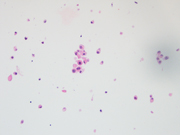
Figure 2: FNA of periumbilical subcutaneous mass, conventional smear, Pap stained, 400x
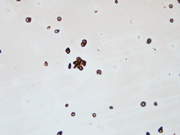
Figure 3: FNA of periumbilical subcutaneous mass, cell block, H&E stained, 400x
Figure 4: FNA of periumbilical subcutaneous mass, cell block, IHC, cytokeratin cocktail, 400x
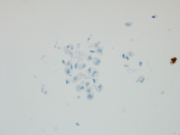
Figure 5: FNA of periumbilical subcutaneous mass, cell block, IHC, D2-40, 400x
Figure 6: FNA of periumbilical subcutaneous mass, cell block, IHC, WT1/EMA, 400x
Figure 7: FNA of periumbilical subcutaneous mass, cell block, IHC, BAP-1, 400x
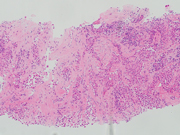
Figure 8: Biopsy of periumbilical subcutaneous mass, H&E stained, 100x
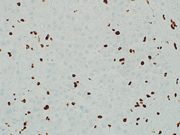
Figure 9: Biopsy of periumbilical subcutaneous mass, IHC, BAP-1, 400x
Questions:
- Based on the clinical history, cytomorphological findings, and immunoprofile, which of the following is the most appropriate diagnosis?
- Mesothelioma
- Reactive mesothelial proliferation
- Adenocarcinoma of Mullerian origin
- Carcinoma of unknown primary
- Melanoma
- Which of the following immunohistochemical markers (IHCs) is the most specific for differentiating mesothelioma from a reactive mesothelial hyperplasia
- Desmin (loss)
- D2-40
- WT1
- EMA
- BAP-1 (loss)
Answers:
Question 1: Correct answer is A
Both Diff Quik- and Pap-stained conventional smears prepared from the FNA of patient’s periumbilical mass showed a cellular aspirate with predominantly single dispersed plasmacytoid-appearing malignant cells, along with occasional loose papillary clusters. Individual cells exhibited a wide variation in size and shape, with scattered enormous cells appreciated. Additionally, bi- and/or multi-nucleation, coarse chromatin, and prominent nucleoli were small cytoplasmic vacuoles and intranuclear inclusions were occasionally seen (Figure 1 and 2). Similar cells were observed in the cell block material (Figure 3). (Figure 7). In addition, the malignant cells were positive for CK7 while negative for calretinin, Wt-1, claudin-4, CK20, p40, INSM1, chromogranin A, synaptophysin, CEA, calcitonin, TTF-1, GATA-3, TRPS1, CDX-2, PAX-8, SOX-10 and Melan-A. Based on the overall findings, a final diagnosis of positive for malignancy was rendered with malignant mesothelioma included in differential diagnoses.
Subsequently, a tissue biopsy of the periumbilical mass was performed and showed clusters of malignant epithelioid cells infiltrating the fibrous tissue (Figure 8). Immunohistochemical stains were performed and showed the malignant cells were positive for cytokeratin cocktail, D2-40, WT-1 and EMA (with an enhanced membranous staining pattern) while negative for Desmin. Further, nuclear staining of BAP1 was absent (Fig 9), while the expression of MTAP and Merlin were retained. The overall findings confirmed the diagnosis of epithelioid mesothelioma.We report a case of peritoneal mesothelioma in a patient who presented with a periumbilical mass at the site of a previously repaired hernia, without concurrent body cavity effusions. In 2020, Sugarbaker reported a similar case involving a 70-year-old woman who presented with a 11 cm abdominal mass within a spigelian hernia defect. The histologic diagnosis of peritoneal mesothelioma was established upon pathological exam of the resected mass.(1) Sugarbaker had previously reported his institutional experience of a new onset hernia as the first presenting clinical finding of peritoneal mesothelioma in 13% of patients.(2)
In the current case, FNA specimen obtained from the patient’s periumbilical mass showed a cellular aspirate with predominantly plasmacytoid-appearing malignant cells, arranged as predominantly dispersed single cells with occasional loose papillary clusters. The characteristic morphology of effusion mesothelioma has been described in details by Michael.(3) Plasmacytoid cells are not commonly seen in mesotheliomas encountered in effusions. Given the patient’s unusual clinical presentation for peritoneal mesothelioma, the differential diagnoses for neoplasms with plasmacytoid-type cells on cytology was broad, which included, but not limited to metastatic carcinoma (i.e. lobular type breast carcinoma), carcinoid tumor, medullary thyroid carcinoma, melanoma and plasmacytoma. Breast origin is unlikely since the malignant cells are negative for GATA-3 and TRPS1. The lack of staining for neuroendocrine markers (INSM1, chromogranin A, and synaptophysin) did not support carcinoid tumor or medullary thyroid carcinoma. Absence of staining for Melan A and SOX-10 made melanoma an unlikely diagnosis. Furthermore, the malignant cells demonstrated Taken together, the overall findings were most consistent with mesothelioma.
This case highlights that mesothelioma should be considered in the differential diagnosis when a patient with a history of hernia or hernial repair presents with a mass or nodule. Given the uncommon clinical presentation, immunohistochemical studies, including those for mesothelioma, should be included during the workup.
Question 2: Correct answer is E
BAP1 nuclear expression loss is observed in 50-76% of peritoneal mesothelioma cases, while complete MTAP cytoplasmic loss is seen in 17% of cases.(4) The 2021 WHO Classification of Tumors of the Pleura has recognized BAP1, EZH2, and MTAP (a surrogate for CDKN2A deletion) immunohistochemistry as an useful tool in distinguishing benign mesothelial proliferations from mesothelioma.(5). EMA may show an enhanced membranous staining pattern in mesothelioma while negative in reactive mesothelial cells. On the contrary, desmin may highlight reactive mesothelial cells while negative in mesothelioma (6). Both reactive mesothelial cells and mesothelioma cells can be positive for D2-40 and/or WT-1.References:
Sugarbaker PH. Malignant peritoneal mesothelioma presenting as a mass in a Spigelian hernia. Report of a case. Int J Surg Case Rep. 2020;68:239-41.
Sugarbaker PH, Welch LS, Mohamed F, Glehen O. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am. 2003;12(3):605-21, xi.
Michael CW. The cytologic diagnosis of mesothelioma: are we there yet? J Am Soc Cytopathol. 2023;12(2):89-104.
Malpica A. Peritoneal Mesothelioma-An Update. Adv Anat Pathol. 2023;30(4):262-74.
Sauter JL, Dacic S, Galateau-Salle F, Attanoos RL, Butnor KJ, Churg A, et al. The 2021 WHO Classification of Tumors of the Pleura: Advances Since the 2015 Classification. J Thorac Oncol. 2022;17(5):608-22.
Hasteh F, Lin GY, Weidner N, Michael CW. The use of immunohistochemistry to distingush reactive mesothelial clls from malignant mesothelioma in cytologic effusions. Cancer Cytopathology. 2010; 118:90-6.