Case of the Month ...
A 79-year-old male presented for routine annual exam and was found to be a compound heterozygote for hereditary hemochromatosis. He subsequently underwent MRI abdomen for liver evaluation, which revealed an incidentally discovered 1.5 cm cyst in the pancreatic tail. Throughout a multi-year course of imaging surveillance, the pancreatic tail cyst grew to 3.7 cm. Upper endoscopy with ultrasound (EUS) was performed and detailed a unilocular pancreatic tail cyst with a thin outer wall, no internal debris and no associated mass lesion. There were no high-risk imaging features. Cyst fluid was aspirated at the time of EUS and sent for cytology and cyst fluid analysis. Cyst fluid analysis revealed a cyst glucose <2, CEA 467, amylase 253.
Authors
- Kelsey McHugh, MD – Mayo Clinic Arizona
Diagnosis & Discussion
click on image for larger version
Figure 3 Figure 4 Figure 5 Figure 6 Images 1-6:
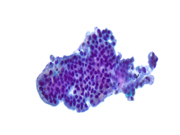
Figure 1: EUS FNA of pancreatic tail cyst, ThinPrep, 200x magnification
Figure 2: EUS FNA of pancreatic tail cyst, ThinPrep, 400x magnification
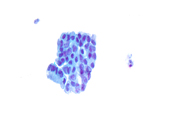
Figure 3: EUS FNA of pancreatic tail cyst, ThinPrep, 400x magnification
Figure 4: EUS FNA of pancreatic tail cyst, ThinPrep, 400x magnification
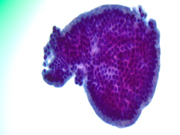
Figure 5: EUS FNA of pancreatic tail cyst, ThinPrep, 400x magnification
Figure 6: EUS FNA of pancreatic tail cyst, ThinPrep, 400x magnificationQuestions:
- What is the best diagnosis based on the cytomorphology in Figures 1 through 6?
- Adenocarcinoma
- Serous cystadenoma
- Neoplastic mucinous cyst with high-grade dysplasia
- Pseudocyst
- Neoplastic mucinous cyst with low-grade dysplasia
- Which of the following is not a cytologic feature of high-grade atypia/dysplasia in pancreatic neoplastic mucinous cyst fluid?
- Cell size equal to that of benign enterocytes (12 mm)
- Increased nuclear-to-cytoplasmic ratio
- Irregular nuclear membrane contours
- Chromatin alterations including hypochromasia and hyperchromasia
- Necrosis
- Which of the following intraductal papillary mucinous neoplasm (IPMN) epithelial lining subtypes most typically show high-grade atypia/dysplasia?
- Gastric-type and intestinal-type
- Seromucinous-type and acinar-type
- Pancreatobiliary-type and gastric-type
- Intestinal-type and pancreatobiliary-type
- Acinar-type and intestinal-type
Answers:
Question 1: Correct answer is C
Neoplastic mucinous cysts of the pancreas generally encompass two entities: intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN). IPMN is far more common than MCN. IPMNs generally communicate with either the main pancreatic duct or a side branch pancreatic duct, whereas MCNs do not communicate with the pancreatic duct. The incidence of neoplastic mucinous cysts of the pancreas is rising, as the frequency of incidentally discovered lesions increases alongside the increasing rate of abdominal imaging. The cytologic features of both IPMN and MCN are essentially indistinguishable. A diagnostic schema for pancreatobiliary cytopathology reporting was first put forth by the Papanicolaou Society of Cytopathology (PSC) in 2015 when they published the PSC System for Reporting Pancreatobiliary Cytology. In this reporting system, neoplastic mucinous cysts fall under the diagnostic umbrella of “neoplastic: other” and diagnostic cytologic features include identification of abundant thick colloid-like extracellular mucin, neoplastic mucinous epithelial cells, and/or elevated cyst fluid CEA or KRAS/GNAS mutation. More recently, the World Health Organization (WHO) in partnership with International Agency for Research on Cancer (IARC), published a new system for reporting pancreatobiliary cytopathology in 2022. Within this new reporting system, two new diagnostic categories were introduced that capture these neoplastic mucinous cysts of the pancreas. These two diagnostic categories, “pancreatobiliary neoplasm, low-risk/grade (PaN-low)” and “pancreatobiliary neoplasm, high-risk/grade (PaN-high)”, mirror the two-tiered histopathologic classification of these cystic neoplasms initially put forth by the 5th Edition WHO Classification of Digestive Tract Tumours.
Of course, there are many challenges to rendering a diagnosis of neoplastic mucinous cyst in the pancreas as well as grading its neoplastic epithelial cells. Issues that arise include lack of clarity in features that help distinguish gastrointestinal contaminating mucin from neoplastic mucin, cytology overlap of contaminating gastric foveolar epithelium and neoplastic gastric-type epithelium with low-grade dysplasia, rounding of single cells suspended with cyst mucin resulting in false positive interpretations for high-grade atypia/dysplasia, and lack of significant cytomorphologic features that help distinguish high grade aytpia/dysplasia from invasive adenocarcinoma within these neoplastic mucinous cysts. Sigel et al did find that the presence of colloid-like mucin, mucin covering at least 20% of the smear surface area, and the presence of histiocytes were all statistically significantly more likely to be seen in neoplastic mucinous cysts than in samples of gastrointestinal contamination. When it comes to discerning low-grade atypia/dysplasia from high-grade atypia/dysplasia within a neoplastic mucinous cysts, identifying four or more cytopathologic features prototypical of high-grade atypia/dysplasia, as described below, has a low sensitivity, but a high specificity for the diagnosis of a high-grade neoplastic mucinous cyst.Question 2: Correct answer is A
Multiple authors (Pitman MB et al; Sigel C et al) have shown that there are a handful of cytologic features that are statically significantly more likely in high grade atypia/dysplasia than in low grade atypia/dysplasia within pancreatic neoplastic mucinous cyst fluid. These cytologic features include: 1. small cell size; 2. increased nuclear-to-cytoplasmic (N/C) ratio; 3. marked nuclear membrane irregularity; 4. abnormal chromatin pattern (hypochromasia or hyperchromasia); 5. background cellular necrosis; 6. three-dimensional architecture; 7. loss of nuclear polarity; 8. >4:1 nuclear size variation in one cell cluster; 9. karyorrhexis; 10. single cells; 11. variability in amount of cytoplasm. Generally, cell size equal to that of a benign enterocyte (approximately 12 mm) is a feature typical of pancreatic neoplastic mucinous cysts with low-grade atypia/dysplasia and these dysplsatic cells can be seen in small tissue fragments or singly dispersed. As highlighted by Sigel et al., the cellularity from low-grade atypia/dysplasia is statistically significantly more likely to be indistinguishable from non-neoplastic gastric contamination than the cellularity of cases with high-grade atypia/dysplasia, highlighting the similarity in cell size and tissue architecture between non-neoplastic gastric foveolar-type epithelium and the epithelium found in many neoplastic mucinous cysts with low-grade atypia/dysplasia, especially those of the gastric epithelial subtype.Question 3: Correct answer is D
The three epithelial lining subtypes of IPMN are: gastric, intestinal and pancreatobiliary. All neoplastic mucinous cysts, including main duct and side branch IPMNs, are now graded using a two-tiered grading system of low-grade atypia/dysplasia and high-grade atypia/dysplasia. Collapse of the previous three-tiered grading system (low, intermediate and high) into this two-tiered system has resulted in lesions previously called low- and intermediate-grade now being labeled as low-grade atypia/dysplasia, with high-grade atypia/dysplasia remaining as its own distinct diagnostic category. Grading of neoplastic mucinous cysts is based on any amount of the highest grade of epithelium identified, generally utilizing a combination of cytomorphology and architectural complexity to generate a final grade. While gastric-type IPMNs typically reveal low-grade atypia/dysplasia in the majority of cases, both intestinal-type IPMNs and pancreatobiliary-type IPMNs are very likely to demonstrate high-grade atypia/dysplasia, with nearly all pancreatobiliary-type IPMNs being classified as high-grade. Other changes that have been made to the IPMN subtyping and grading systems include the elimination of oncocytic as an IPMN epithelial lining subtype. Rather, what was previously termed an oncocytic-type IPMN is now its own entity, called intraductal oncocytic papillary neoplasm (IOPN). This change was made given these lesion have a histomorphology, molecular profile, and tumor biology distinct from IPMNs. Most notably regarding the molecular biology of IOPNs, these tumors lack the KRAS and GNAS mutations typical of IPMNs, more frequently harboring rearrangements in PRKACA and PRKACB, and mutations in ARHGAP26, ASXL1, EPHA8, and ERBB4.References:
Pitman MB, Layfield LJ. Guidelines for pancreaticobiliary cytology from the Papanicolaou Society of Cytopathology: A review. Cancer Cytopathol. 2014;122:399-411.
The Papanicolaou Society of Cytopathology System for Reporting Pancreatobiliary Cytology: Definitions, Criteria and Explanatory Notes. Springer Nature. 2015; 1st ed.
International Academy of Cytology – International Agency for Research on Cancer – World Health Organization Joint Editorial Board. WHO Reporting System for Pancreaticobiliary Cytopathology [Internet]. Lyon (France): International Agency for Research on Cancer; 2022 [cited 2024/09/04]. (IAC-IARC-WHO cytopathology reporting systems series, 1st ed.; vol. 2). Available from: https://tumourclassification.iarc.who.int/chapters/50.
Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino-Kenudson M. Cytologic Criteria of High-Grade Epithelial Atypia in the Cyst Fluid of Pancreatic Intraductal Papillary Mucinous Neoplasms. Cancer Cytopathol. 2014;122(1):40-47.
Sigel C, Wei XJ, Agaram N, et al. Diagnostic features of low- and high-grade mucinous neoplasms in pancreatic cyst FNA cytology. Cancer Cytopathol. 2023;131:325-336.
Salla C, Karvouni E, Nikas I, et al. Imaging and Cytopathological Criteria Indicating Malignancy in Mucin-Producing Pancreatic Neoplasms: A Series of 68 Histopathologically Confirmed Cases. Pancreas. 2018;47:1283-1289.
Wehrle CJ, Hossain MS, Perlmutter B, et al. Consequences of a Surveillance Strategy for Side-branch Intraductal Pancreatic Mucinous Neoplasms: Long-term Follow-up of One Thousand Cysts. Ann Surg. 2024;280:683-692.